Abstract
Background
In the UK, during the study period (April to July, 2021), all contacts of people with COVID-19 were required to self-isolate for 10 days, which had adverse impacts on individuals and society. Avoiding the need to self-isolate for those who remain uninfected would be beneficial. We investigated whether daily use of lateral flow devices (LFDs) to test for SARS-CoV-2, with removal of self-isolation for 24 h if negative, could be a safe alternative to self-isolation as a means to minimise onward transmission of the virus.
Methods
We conducted a randomised, controlled, non-inferiority trial in adult contacts identified by COVID-19 contact tracing in England. Consenting participants were randomly assigned to self-isolation (single PCR test, 10-day isolation) or daily contact testing (DCT; seven LFD tests, two PCR tests, no isolation if negative on LFD); participants from a single household were assigned to the same group. Participants were prospectively followed up, with the effect of each intervention on onward transmission established from routinely collected NHS Test and Trace contact tracing data for participants who tested PCR-positive for SARS-CoV-2 during the study period and tertiary cases arising from their contacts (ie, secondary contacts). The primary outcome of the study was the attack rate, the percentage of secondary contacts (close contacts of SARS-CoV-2-positive study participants) who became COVID-19 cases (tertiary cases) in each group. Attack rates were derived from Bernoulli regression models using Huber-White (robust) sandwich estimator clustered standard errors. Attack rates were adjusted for household exposure, vaccination status, and ability to work from home. The non-inferiority margin was 1·9%. The primary analysis was a modified intention-to-treat analysis excluding those who actively withdrew from the study as data from these participants were no longer held. This study is registered with the Research Registry (number 6809). Data collection is complete; analysis is ongoing.
Findings
Between April 29 and July 28, 2021, 54 923 eligible individuals were enrolled in the study, with final group allocations (following withdrawals) of 26 123 (52·6%) participants in the DCT group and 23 500 (47·4%) in the self-isolation group. Overall, 4694 participants tested positive for SARS-CoV-2 by PCR (secondary cases), 2364 (10·1%) in the self-isolation group and 2330 (8·9%) in the DCT group. Adjusted attack rates (among secondary contacts) were 7·5% in the self-isolation group and 6·3% in the DCT group (difference of –1·2% [95% CI –2·3 to –0·2]; significantly lower than the non-inferiority margin of 1·9%).
Interpretation
DCT with 24 h exemption from self-isolation for essential activities appears to be non-inferior to self-isolation. This study, which provided evidence for the UK Government's daily lateral flow testing policy for vaccinated contacts of COVID-19 cases, indicated that daily testing with LFDs could allow individuals to reduce the risk of onward transmission while minimising the adverse effects of self-isolation. Although contacts in England are no longer required to isolate, the findings will be relevant for future policy decisions around COVID-19 or other communicable infections.
Funding
UK Government Department of Health and Social Care.
Introduction
In England, the NHS Test and Trace (NHSTT) programme has provided access to testing and contact tracing for close contacts of individuals with confirmed COVID-19 (COVID-19 cases).1 At the time of this study (April 29 to July 28, 2021), all contacts, vaccinated and unvaccinated, were required to isolate for 10 days from the date of exposure to the primary case. From Aug 22, 2021, vaccinated contacts and individuals younger than 18·5 years were no longer required to isolate, but unvaccinated adult contacts were required by legislation to self-isolate for 10 days from the date of their last exposure to the case. From Feb 18, 2021, all COVID-19 contacts were offered a single PCR test, irrespective of symptoms;2 however, only half of identified contacts performed this test. Most contacts of COVID-19 cases modify their behaviours and contact with other people. However, full adherence to self-isolation guidance in England remained between 50% and 90% (up to August, 2021),3, 4, 5 reducing the effectiveness of isolation on viral transmission. Strategies to improve self-isolation compliance have been developed, including provision of financial or other incentives and penalties;6 however, such strategies do not consider the wider economic, social, and wellbeing impacts of self-isolation.7
Research in context.
Evidence before this study
We searched Ovid MEDLINE, with no language restrictions, for randomised controlled trials published from database inception up to Jan 12, 2022. We used a combination of MeSH and free-text terms including those for SARS-CoV-2 or COVID-19 combined with terms for lateral flow testing (eg, “lateral flow” or “rapid test*” or “antigen* test*” or “daily adj3 test*”). We found no existing trials on the use of daily lateral flow device (LFD) testing as an alternative to self-isolation for contacts of confirmed COVID-19 cases among the general population. Two previous feasibility and acceptability studies performed by the study team have demonstrated the potential benefits of daily testing of adult contacts of COVID-19 cases using either a single PCR test or a test-to-enable approach using daily LFD tests plus one PCR test as part of the contact tracing process in England. However, these studies were not designed to assess the risk of onward transmission.
Added value of this study
To our knowledge, we present the first randomised controlled trial in a general population of close contacts of COVID-19 cases to assess whether daily LFDs with 24 h exemption from self-isolation for essential activities following each negative LFD result is a safe alternative to self-isolation. By ascertaining the proportion of contacts of PCR-positive study participants who became tertiary cases in each group, we showed that daily contact testing (DCT) was a safe alternative to self-isolation regarding onward transmission of SARS-CoV-2, with a difference in attack rates of –1·2% among contacts of secondary cases, indicating non-inferiority of DCT. Consistent with this finding, the number of observed tertiary cases per secondary case was 0·14 in the DCT group and 0·16 in the self-isolation group. Attack rates among contacts of DCT participants who had received two vaccine doses (>14 days before recruitment) also showed non-inferiority compared with self-isolation-participants, suggesting that DCT would be of value among fully vaccinated people. Compliance with testing was high, with 80% of DCT participants submitting at least one LFD result.
Implications of all the available evidence
The COVID-19 pandemic continues to have a large impact on individuals and society. Developing public health interventions that mitigate both viral transmission and the wider impacts on health, wellbeing, prosperity, and society, including those arising from self-isolation, is essential. Our study demonstrates the potential benefits of daily testing while minimising the need for self-isolation. A cluster-randomised controlled trial conducted in English secondary schools and colleges conducted at the same time as this study found supervised daily LFD tests to be non-inferior to self-isolation for control of COVID-19 transmission in those settings, although the study did not measure secondary transmission. In December, 2021, based on evidence from both our study and the school-based study, the UK Government introduced daily lateral flow testing for fully (two-dose) vaccinated contacts of confirmed COVID-19 cases as an alternative to isolation. Assessment of the impact of this policy change on onward transmission of SARS-CoV-2 would provide further, real-world, evidence of the benefits and any potential harms of this strategy when used at national level.
Strategies that target self-isolation more effectively to contacts who become infected, while allowing those without infection to continue with essential activities, would help individuals and society to return to greater normality while continuing to reduce onward transmission. Improving case ascertainment through the identification of asymptomatic, paucisymptomatic, and presympromatic individuals could help to target isolation most effectively and, potentially, improve adherence to self-isolation guidance.8, 9, 10 Asymptomatic, rapid antigenic testing using lateral flow devices (LFDs) for SARS-CoV-2 was widely available in the UK during the course of our study,11, 12 with low cost, rapid turnaround times, and delivery outside of a routine laboratory environment. We considered that such tests could be suitable to support a structured programme of testing contacts of cases.8, 9, 10
Two previous feasibility and acceptability studies showed the potential benefits of a structured programme of daily testing of contacts with either a single PCR test13 or a test-to-enable approach using daily LFD tests plus one PCR test as part of the contact tracing process in England.14, 15, 16, 17 However, these studies were not designed to assess the risk of onward transmission. The use of daily contact testing (DCT) using LFD tests as an alternative to self-isolaton was explored in a school-based study, which reported that the use of daily LFD tests was non-inferior to self-isolation for control of SARS-CoV-2 transmission.18 Here we report the results of a randomised, controlled, non-inferiority trial of adult, close contacts of COVID-19 cases to test whether using daily LFD tests with 24 h exemption from self-isolation following each negative LFD result, in combination with two PCR tests, was a safe alternative to self-isolation combined with a single PCR test.
Methods
Study design and participants
This study was a two-arm, non-blinded, randomised, controlled, non-inferiority trial using a non-inferiority margin of 1·9%. This margin was derived from a previous, much smaller, pilot study14 in which tertiary attack rates were 6·3% in the DCT group and 8·2% in the general population. Lower tertiary attack rates in the DCT group were the opposite of what was expected, but the study team considered that a 1·9% higher percentage positive for DCT compared with self-isolation would indicate that DCT could be considered, in terms of infection control consequence, as not being inferior to self-isolation in the current study. Adults (≥18 years) who were vaccinated or unvaccinated against SARS-CoV-2, identified as contacts of confirmed COVID-19 cases, and living in England, were offered participation in the study (appendix pp 2–10). Participants were not eligible to participate if they met the following criteria: symptomatic at recruitment; under travel-associated quarantine; participating in a workplace DCT programme; resident in a prison or social care institution; a contact of a case with a variant of concern (between April 29 and June 7, 2021, only; removed after this date to ensure generalisability for the delta variant); or did not provide an email address. Participants were also excluded if no postage address was provided. If a participant had duplicate registrations (with an alternative contact tracing identification number) within 3 days from first registration, the duplicate registration was excluded.
Individuals were recruited through the routine NHSTT contact tracing process and selected sequentially. Information on eligibility criteria was self-reported. Self-reported age, postcode, travel criteria, and whether they were a contact of a case with a variant of concern were confirmed using data collected by NHSTT during contact tracing. Recruitment was performed daily from April 29, 2021, until the sample size was reached (July 28, 2021), with eligible contacts invited to take part via recruitment phone calls or via SMS or emails containing a link to self-register online. There was no limit on daily recruitment; however, due to intermittent limitations in the number of study kits available, enrolment was restricted on June 26–29, July 3–12, and July 19–26, 2021. A subset of participants were interviewed after completing testing or self-isolation as part of a nested qualitative component to the study, which is reported elsewhere.19
Ethics approval was granted by the UK Health Security Agency (UKHSA; formerly Public Health England) Research Ethics and Governance Group (reference NR0235). Written informed consent was obtained during recruitment.
Randomisation
Participants were randomly assigned to either the DCT group (daily contact testing with seven self-administered LFD tests, with release for 24 h based on a negative LFD result) or the standard isolation group (a single self-taken PCR swab and self-isolation for 10 days). Simple randomisation without stratification was performed using an unpredictable allocation sequence at the point of consent based on a computer system-generated timestamp for each participant, with allocation generated by the study team and concealed from individuals performing recruitment. Contacts from the same household were reassigned to the group of the first member of the household recruited, irrespective of the outcome of randomisation, with clustering accounted for in the analysis.
Procedures
Demographic data were collected at recruitment using a secure electronic questionnaire (Snap 11 Professional; Snap Surveys, London, UK) and downloaded twice daily to produce lists for test kit postage and messaging. Kits were posted via NHSTT home delivery channels. Within 24 h of recruitment, participants with a valid mobile number or email address were informed of their assigned study group and sent a link to a short, voluntary, anonymous baseline questionnaire. On day 7, a link was sent with a completion-of-study questionnaire. Reminder messages were sent after 48 h.
Participants in both groups were asked to take a self-sample for PCR on the day of kit arrival and return it by post. DCT participants were provided with seven Innova LFD tests (Innova SARS-CoV-2 Rapid Antigen Test; (Xiamen Biotime Biotechnology, Fujian, China), which were widely used in the UK at the time of the study; participants were required to self-process tests using combined nasal and oropharyngeal swabs. Previous studies have estimated the sensitivity of this kit as being between 57·5% and 90·9%.20, 21 DCT participants were asked to perform their first LFD test on the day of kit arrival and then on each of the following 6 days, reporting results daily to a secure study portal (developed in Snap Surveys). Reported results were submitted to the national results database in compliance with infectious disease notification regulations. On reporting their first negative LFD test, DCT participants were assigned a flag in the NHSTT contact tracing system to prevent isolation checks and access to self-isolation support payments. A second PCR swab was requested for DCT participants on receipt of an LFD-positive result or on the day of their last LFD test (if all previous LFD tests were negative). All participants were legally required to self-isolate for 10 days if PCR-positive. No formal restrictions were placed on study participants in the DCT group during periods free from self-isolation; however, participants were advised to minimise contact and undertake only essential activities.
Outcomes
The primary outcome was the attack rate, defined as the percentage of secondary contacts (close contacts of SARS-CoV-2-positive study participants) who became COVID-19 cases (tertiary cases) in each group. The aim was to establish whether DCT was non-inferior to self-isolation, using a non-inferiority margin of 1·9% of the difference in percentages (self-isolation minus DCT).
Secondary objectives of the study were to establish the feasibility and acceptability of each strategy by measuring uptake and compliance with testing, ascertaining the proportion of positive results, and describing the concordance of PCR and LFD results. These secondary outcomes will be reported separately in full. A further prespecified secondary outcome, described in the present report, was the number of days of exemption from isolation enabled by DCT. The present report also describes findings from the end-of-study questionnaire, which inquired about participant behaviours during the study period and participants' confidence in test accuracy. Results from an in-depth qualitative study performed with a subset of participants are published separately.19
Statistical analysis
With allowance for attrition and testing compliance, 40 000 participants were required to generate 3170 secondary contacts based on a two-sided non-inferiority sample size calculation with a significance level of 0·05, power of 80%, ratio of group sizes 1:1, design effect of 1·2, and a non-inferiority margin of 1·9%, using the ARTBIN22 package in Stata (version 16.1). At the study midpoint, the sample size was inflated to 50 000 to account for a lower-than-anticipated detection of SARS-CoV-2 in primary contacts, suspected to be related to vaccination.
Data submitted to the study LFD portal and recruitment portal were analysed as of Aug 14, 2021, with PCR data analysed as of Sept 8, 2021. Data were analysed in Stata (version 15) and R Studio (version 4.0.5). Recruitment data were enriched using routinely collected NHSTT contact tracing data and deterministically linked to PCR results from national laboratory surveillance, study LFD results, and the national LFD result portal, and to immunisation data from the National Immunisation Management System (NIMS) using a combination of identifiers. PCR results from all participants were restricted to tests with a specimen date in the 90 days before recruitment (to adjust for extended PCR positivity) to 14 days after recruitment. Fully vaccinated and one-dose vaccinated individuals were defined as those vaccinated more than 14 days before recruitment. When NIMS vaccination status was unknown, self-reported vaccination status was used.
Attack rates were derived from participants (primary contacts) who tested positive for SARS-CoV-2 by PCR in the 2 days before and 14 days after recruitment (secondary cases). Participants were deterministically linked to case episodes in the NHSTT case management system, and their named close contacts identified. Potential transmission events were defined as contact records matched to a subsequent case record with symptom onset (test date if asymptomatic) between 2 days and 14 days (inclusive) after the exposure date. When the contact was in the household, the date of symptom onset (test date if asymptomatic) of the exposer was taken as the exposure date. When multiple case–contact exposures could have resulted in transmission, rules-based prioritisation (preferring household exposures and most recent exposures) identified a single most likely potential transmission event. The attack rate was the proportion of contacts of participants (secondary contacts) that were identified as potential transmission events, leading to tertiary cases. Bernoulli regression models using Huber-White (robust) sandwich estimator clustered standard errors were used to account for the inflation due to participants being grouped within households. These were calculated in R (version 4.0.5) using the glm.cluster command from the miceadds package23 and the predictions and margins commands from the margins package. The primary analysis was a modified intention-to-treat analysis excluding those who actively withdrew from the study as data from these participants were no longer held (as per the public-facing data agreement). Those who did not submit a test were included in the analysis. The simplest unadjusted model used group as the only covariate. The second unadjusted model added household exposure and its interaction with group, while the third unadjusted model instead added vaccine status (zero or one doses; two doses) and its interaction. Adjusted versions of these models were obtained by adding household exposure, vaccine status, and ability to work from home. Interactions were tested for significance by Wald tests with a significance level of 0·05. Sensitivity analyses restricted to (1) DCT participants who submitted LFD results to the study portal and self-isolation group participants who did not report LFD results to the study portal (as a proxy for compliance) and (2) the first household member recruited (to account for allocation to the same group for multiple household members), and with both restrictions, were performed. Alternative mixed effects models with household as random effect were performed and an independent unadjusted masked analysis was also performed. Full code can be provided on request.
For both behavioural questionnaires, proportions were calculated among participants who provided a least one response to a question, and were compared using χ2 tests. The second behavioural questionnaire was analysed as three groups: participants in the self-isolation group; participants in the DCT group who tested positive on LFD (hereafter referred to as DCT positive-test subgroup); and participants in the DCT group who did not test positive on LFD (hereafter referred to as DCT no-positive-test subgroup).
The trial was registered with the Research Registry (number 6809).24
Role of the funding source
The UK Government Department of Health and Social Care sponsored the trial and was involved in study design and logistical support for PCR and LFD testing. Otherwise, the study sponsor had no role in data analysis, data interpretation, or writing of the report.
Results
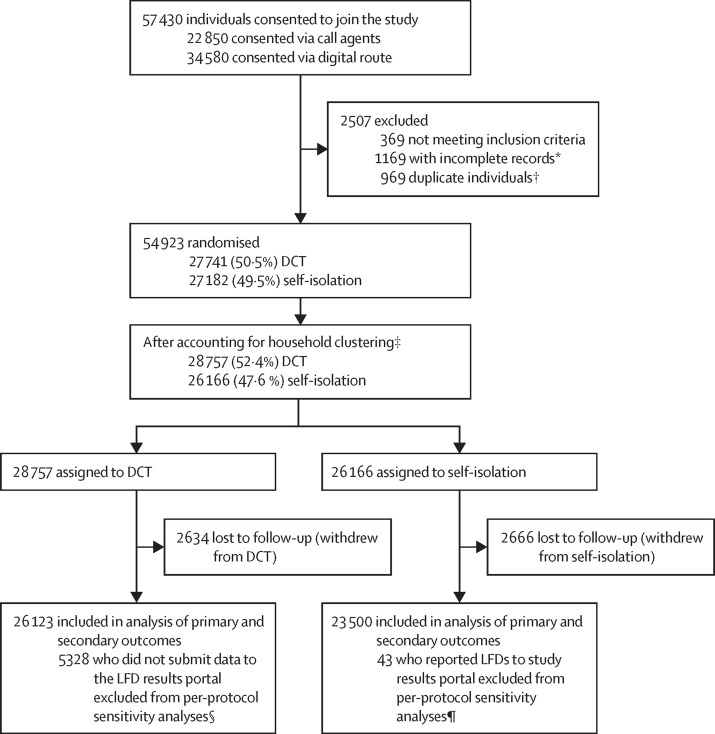
Between April 29 and July 28, 2021, 57 430 unique contacts of confirmed cases of COVID-19 consented to participation in the study (figure ). 34 580 (60·2%) participants self-enrolled digitally and 22 850 (39·8%) enrolled via telephone calls. 54 923 (95·6%) of 57 430 consenting individuals were eligible for inclusion (1169 had no address or contact information, 369 met exclusion criteria, and 969 were duplicate participants). 27 741 (50·5%) participants were randomly assigned to the DCT group and 27 182 (49·5%) to the self-isolation group. 5300 participants withdrew after randomisation (2634 [49·7%] DCT participants and 2666 [50·3%] self-isolation participants); common reasons for withdrawing were dissatisfaction with group allocation (n=1453), being at the end of isolation (n=770), having a previous PCR test (n=568), and already testing positive (n=453; appendix p 11). Household members were assigned to the same study group after randomisation, with final group allocations being 26 123 (52·6%) DCT participants and 23 500 (47·4%) self-isolation participants.
Figure.
Trial profile
DCT=daily contact testing. LFD=lateral flow device. *Incomplete records were excluded following recruitment as no address or contact information was provided. †Individuals registering more than once with the same NHS Test and Trace identification number were identified as duplicates, with only the first registration retained. ‡If multiple contacts were reported from a single household (participants with the same postcode and house number were grouped as household members), then all individuals in the household were assigned to the same group of the study after recruitment, with all individuals assigned to the group to which the first member of the household recruited was assigned. §5328 participants were excluded from per-protocol sensitivity analyses in the DCT group as they had not submitted results to the LFD portal and it was not possible to verify that they had participated in the study. ¶43 participants were excluded from per-protocol sensitivity analyses in the self-isolation group as it could not be verified that they had followed self-isolation as required.
There were no differences in the sex, age, regional distribution, ethnicity, vaccination status, or the presence of a COVID-19 case in the household between the two groups (table 1 ). DCT participants were less likely to work outside of the home than self-isolation participants (40·1% vs 43·2%). 41·7% of DCT participants and 36·1% of self-isolation participants had more than one household member in the study or registered more than once during recruitment.
Table 1.
Baseline characteristics of participants by study group
| Self-isolation group (n=23 500) | DCT group (n=26 123) | ||
|---|---|---|---|
| Age, years | 43 (30–52) | 43 (29–53) | |
| Sex | |||
| Female | 12 734 (54·2% [53·6–54·8]) | 14 000 (53·6% [53·0–54·2]) | |
| Male | 10 756 (45·8% [45·2–46·4]) | 12 113 (46·4% [45·8–47]) | |
| Geography | |||
| East Midlands | 1635 (7·0% [6·6–7·3]) | 1822 (7·0% [6·7–7·3]) | |
| East of England | 2086 (8·9% [8·5–9·2]) | 2158 (8·3% [7·9–8·6]) | |
| London | 2627 (11·2% [10·8–11·6]) | 2947 (11·3% [10·9–11·7]) | |
| North East | 1992 (8·5% [8·1–8·8]) | 2282 (8·7% [8·4–9·1]) | |
| North West | 4524 (19·3% [18·8–19·8]) | 4917 (18·8% [18·4–19·3]) | |
| South East | 3331 (14·2% [13·7–14·6]) | 3813 (14·6% [14·2–15·0]) | |
| South West | 2446 (10·4% [10–10·8]) | 2782 (10·7% [10·3–11·0]) | |
| West Midlands | 1995 (8·5% [8·1–8·8]) | 2199 (8·4% [8·1–8·8]) | |
| Yorkshire & Humber | 2856 (12·2% [11·7–12·6]) | 3176 (12·2% [11·8–12·6]) | |
| Index of multiple deprivation | |||
| 1—Most deprived | 1403 (6·0% [5·7–6·3]) | 1493 (5·8% [5·5–6]) | |
| 2 | 1589 (6·8% [6·5–7·1]) | 1630 (6·3% [6·0–6·6]) | |
| 3 | 1788 (7·7% [7·3–8]) | 1912 (7·4% [7·0–7·7]) | |
| 4 | 1972 (8·4% [8·1–8·8]) | 2194 (8·4% [8·1–8·8]) | |
| 5 | 2168 (9·3% [8·9–9·7]) | 2521 (9·7% [9·3–10·1]) | |
| 6 | 2418 (10·4% [10–10·7]) | 2672 (10·3% [9·9–10·7]) | |
| 7 | 2745 (11·8% [11·3–12·2]) | 3032 (11·7% [11·3–12·1]) | |
| 8 | 2922 (12·5% [12·1–12·9]) | 3182 (12·3% [11·8–12·6]) | |
| 9 | 2952 (12·6% [12·2–13·1]) | 3357 (12·9% [12·5–13·3]) | |
| 10—Least deprived | 3399 (14·6% [14·1–15]) | 3993 (15·4% [14·9–15·8]) | |
| Ethnicity | |||
| Asian | 826 (3·6% [3·3–3·8]) | 856 (3·3% [3·1–3·5]) | |
| Black | 241 (1·0% [0·9–1·2]) | 264 (1·0% [0·9–1·1]) | |
| Mixed | 569 (2·5% [2·3–2·6]) | 707 (2·7% [2·5–2·9]) | |
| White | 21 346 (91·9% [91·5–92·2]) | 23 718 (91·8% [91·4–92·1]) | |
| Other | 250 (1·1% [0·9–1·2]) | 304 (1·2% [1·0–1·3]) | |
| Vaccination status* | |||
| Unvaccinated | 3022 (13·0% [12·5–13·4]) | 3390 (13·1% [12·7–13·5]) | |
| 1 dose | 5978 (25·7% [25·1–26·2]) | 6343 (24·6% [24–25·1]) | |
| 2 doses | 14 291 (61·4% [60·7–62]) | 16 103 (62·3% [61·7–62·9]) | |
| COVID-19 case in household† | |||
| No | 9376 (40·3% [39·7–41]) | 10 134 (39·3% [38·7–39·9]) | |
| Yes | 13 877 (59·7% [59–60·3]) | 15 666 (60·7% [60·1 − 61·3]) | |
| Homeworker‡ | |||
| No | 10 035 (43·2% [42·6–43·9]) | 10 324 (40·1% [39·5–40·7]) | |
| Yes | 13 175 (56·8% [56·1–57·4]) | 15 424 (59·9% [59·3–60·5]) | |
| Multiple study participants in household§ | |||
| No | 15 006 (63·9% [63·2–64·5]) | 15 239 (58·3% [57·7–58·9]) | |
| Yes | 8494 (36·1% [35·5–36·8]) | 10 884 (41·7% [41·1–42·3]) | |
Data are n (% [95% CI]) or median (IQR). Data completeness for the self-isolation group and the DCT group, respectively, is as follows: for sex, 23 490/23 500 (100%) and 26 113/26 123 (100%); for age, 23 153 (98·5%) and 25 749 (98·6%); for geography (UKHSA region), 23 492 (100%) and 26 096 (99·9%); for index of multiple deprivation, 23 356 (99·4%) and 25 986 (99·5%); for ethnicity, 23 232 (98·9%) and 25 849 (99·0%); for self-reported vaccination status, 23 291 (99·1%) and 25 836 (98·9%); for index case in household, 23 253 (98·9%) and 25 800 (98·8%); for self-reported ability to work from home, 23 210 (98·8%) and 25 748 (98·6%); for more than one household member or an individual registered more than once in the study, 23 500 (100%) and 26 123 (100%).
Self-reported. Question: “Have you received a vaccination for COVID-19”. Options: yes—2 doses; yes—1 dose; no.
Self-reported. Question: “Does the person with COVID-19 that you were exposed to live in your household?”. Options: yes, no.
Self-reported. Question: “Are you able to work from home?”. Options: yes, no.
Derived from house number and postcode given at recruitment. Participants with same postcode and house number grouped as household members; includes individuals registered more than once if more than 3 days from first registration.
Using a modified intention-to-treat model, of the 49 623 participants who did not actively withdraw (primary contacts), 2364 (10·1%) reported at least one positive PCR result in the self-isolation group and 2330 (8·9%) in the DCT group in the period between 2 days before recruitment and 14 days after recruitment, hereafter referred to as secondary cases. These 4694 individuals were linked to 4748 cases in the NHSTT contact tracing database (where a case had multiple records, all were included). 3815 (80·3%) of 4748 cases reported at least one contact (secondary contacts): 1953 (81·7%) of 2390 PCR-positive self-isolation participants and 1862 (79·0%) of 2358 PCR-positive DCT participants (table 2 ). In total, 10 410 secondary contacts were reported; 5219 contacts reported by self-isolation participants and 5191 contacts reported by DCT participants. Of these secondary contacts, 718 became tertiary cases (393 from self-isolation participants and 325 from DCT participants).
Table 2.
Number of PCR-positive participants (secondary cases), their contacts (secondary contacts), and number of tertiary cases identified in NHSTT records
| Self-isolation group | DCT group | Total | |
|---|---|---|---|
| Number of PCR-positive cases among study participants (secondary cases) | 2364 | 2330 | 4694 |
| Number of PCR-positive cases among study participants (secondary cases) identified in NHSTT | 2390 | 2358 | 4748 |
| Number of secondary cases with NHSTT secondary contacts | 1953 | 1862 | 3815 |
| Number of secondary cases with NHSTT household secondary contacts | 1927 | 1823 | 3750 |
| Number of secondary cases with NHSTT non-household secondary contacts | 214 | 233 | 447 |
| Number of secondary contacts | 5219 | 5191 | 10 410 |
| Number of household secondary contacts | 4651 | 4545 | 9196 |
| Number of non-household secondary contacts | 568 | 646 | 1214 |
| Number of tertiary cases | 393 | 325 | 718 |
| Number of tertiary cases from household contacts | 373 | 306 | 679 |
| Number of tertiary cases from non-household contacts | 20 | 19 | 39 |
| Number of secondary contacts per participant case (all cases) | 2·2 | 2·2 | 2·2 |
| Number of secondary contacts per participant case (cases with contacts) | 2·7 | 2·8 | 2·7 |
| Number of household secondary contacts per participant case (all cases) | 2·0 | 1·9 | 1·9 |
| Number of household secondary contacts per participant case (cases with household contacts) | 2·4 | 2·5 | 2·5 |
| Number of non-household secondary contacts per participant case (all cases) | 0·2 | 0·3 | 0·3 |
| Number of non-household secondary contacts per participant case (cases with non-household secondary contacts) | 2·7 | 2·8 | 2·7 |
| Number of tertiary cases per NHSTT secondary case | 0·2 | 0·1 | 0·2 |
| Number of tertiary cases per secondary case via household secondary contact | 0·2 | 0·1 | 0·1 |
| Number of tertiary cases per secondary case via non-household secondary contact | 0·01 | 0·01 | 0·01 |
Where a case had multiple records, all were included. DCT=daily contact testing. NHSTT=NHS Test and Trace.
Overall, 2·19 secondary contacts were reported per secondary case (2·18 per case in the self-isolation group and 2·20 per case in the DCT group; no statistical difference), with the majority of these being household contacts (1·95 household secondary contacts per case in self-isolation group and 1·93 in DCT group). The number of tertiary cases per secondary case was 0·14 in the DCT group and 0·16 in the self-isolation group. Adjusted attack rates among secondary contacts were 7·5% (95% CI 6·7–8·3) in the self-isolation group and 6·3% (5·6–7·0) in the DCT group. The difference in attack rate between the groups was –1·2% (95% CI –2·3 to –0·2). The upper limit of the confidence interval for the difference (ie, –0·2%) was below the non-inferiority margin of (+)1·9%, suggesting that DCT is non-inferior to self-isolation (table 3 ).
Table 3.
Attack rates in secondary contacts and differences in attack rates among secondary contacts
| Unadjusted attack rate (95% CI) | Adjusted attack rate (95% CI) | ||
|---|---|---|---|
| Group (unadjusted, n=10 410; adjusted, n=10 252) | |||
| DCT group | 6·3% (5·6 to 7·0) | 6·3% (5·6 to 7·0) | |
| Self-isolation group | 7·5% (6·8 to 8·3) | 7·5% (6·7 to 8·3) | |
| Difference in attack rate (DCT vs self-isolation groups) | −1·3% (−2·3 to −0·2) | −1·2% (−2·3 to −0·2) | |
| Group and household exposure (unadjusted, n=10 410; adjusted, n=10 252) | |||
| DCT group: household secondary contacts | 6·7% (6·0 to 7·5) | 6·7% (5·9 to 7·5) | |
| Self-isolation group: household secondary contacts | 8·0% (7·2 to 8·9) | 8·0% (7·2 to 8·9) | |
| DCT group: non-household secondary contacts | 2·9% (1·6 to 4·3) | 2·8% (1·4 to 4·1) | |
| Self-isolation group: non-household secondary contacts | 3·5% (1·9 to 5·1) | 3·6% (1·9 to 5·2) | |
| Difference in attack rate | |||
| DCT vs self-isolation: household secondary contacts | −1·3% (−2·4 to −0·1) | −1·3% (−2·4 to −0·1) | |
| DCT vs self-isolation: non-household secondary contacts | −0·6% (−2·7 to 1·6) | −0·8% (−2·9 to 1·3) | |
| Group and vaccination status (unadjusted, n=10 372; adjusted, n=10 252) | |||
| DCT group: 0 or 1 vaccine dose | 6·8% (5·8 to 7·9) | 7·0% (6·0 to 8·0) | |
| Self-isolation group: 0 or 1 vaccine dose | 7·9% (6·8 to 9·0) | 7·9% (6·8 to 9·0) | |
| DCT group: 2 vaccine doses | 5·5% (4·6 to 6·5) | 5·4% (4·5 to 6·4) | |
| Self-isolation group: 2 vaccine doses | 7·0% (5·9 to 8·2) | 7·0% (5·9 to 8·1) | |
| Difference in attack rate | |||
| DCT vs self-isolation: 0 or 1 vaccine dose | −1·0% (−2·5 to 0·5) | −0·9% (−2·4 to 0·6) | |
| DCT vs self-isolation: 2 vaccine doses | −1·5% (−3·0 to −0·01) | −1·6% (−3·1 to −0·1) | |
Unadjusted models include named variables (group, group and household exposure, and group and vaccination status) as covariates. Adjusted versions of these models were obtained by adding all other covariates from household exposure, vaccine status, and ability to work from home. Self-isolation was used as a baseline against which DCT was compared. Model testing for significance of group and household exposure interaction and group and vaccination status interaction were not significant (unadjusted model for group and household exposure: p=0·97; adjusted model for group and household exposure: p=0·81; unadjusted model for group and vaccination status: p=0·56; adjusted model for group and vaccination status: p=0·46). DCT=daily contact testing.
Adjusted attack rates among secondary household contacts of secondary cases were 6·7% in the DCT group and 8·0% in the self-isolation group, although not significantly different (difference in attack rate –1·3% [95% CI –2·4 to –0·1]). Attack rates among non-household secondary contacts did not differ between the DCT group (2·8%) and the self-isolation group (3·6%; difference in attack rate –0·8% [–2·9 to 1·3]). Attack rates did not significantly differ between groups for secondary contacts who were unvaccinated or partially vaccinated (7·0% in DCT group and 7·9% in self-isolation group; difference in attack rate –0·9% [–2·4 to 0·6]). The difference was greater in magnitude, but not significant, for fully vaccinated secondary contacts (5·4% in DCT group vs 7·0% in self-isolation group; difference in attack rate –1·6% [–3·1 to –0·1]). Results from models testing group and household exposure interaction and group and vaccination status interaction were not significant (table 3).
Regarding LFD testing uptake and compliance, between April 30 and Aug 9, 2021, 124 010 unique LFD test results were reported to the study portal from 20 795 (79·6%) DCT participants. 5328 DCT participants did not report LFD results to the study portal, of whom 1300 reported at least one result to the national, non-study portal (appendix p 13). Demographic characteristics differed significantly between people who did and did not report an LFD result to the portal. Individuals in lower index-of-multiple-deprivation deciles, those of minority ethnicity background, and those unable to work from home were less likely to report a result (appendix pp 14–15). 19 663 (94·6%) of 20 795 DCT participants reported only negative or void LFD results and 1132 (5·4%) of 20 795 DCT participants reported at least one positive LFD result.
A sensitivity analysis removed DCT participants who had not submitted an LFD result to the study portal and self-isolation group participants who had reported LFD results to the study portal (appendix pp 16–17), again indicating that DCT was non-inferior to self-isolation (attack rate 7·4% in the self-isolation group and 6·1% in the DCT group). A separate sensitivity analysis that was restricted to the first person recruited in the household (appendix pp 18–19) also indicated that DCT was non-inferior to self-isolation (adjusted attack rate 7·1% in the self-isolation group and 6·3% in the DCT group). Results of a further sensitivity analysis that combined both restrictions, considering only the first person recruited in the household after exclusion of those DCT participants who had not submitted an LFD result to the study portal, was also consistent with results of the main analysis (appendix pp 20–21).
For DCT participants who worked outside of the home and did not test LFD-positive or PCR-positive during their testing period (n=7457), the number of days free from self-isolation and therefore the number of work days enabled through DCT were estimated at up to 44 089 days (mean of 5·9 days per participant who worked outside of the home [SD 1·79]). Overall, the number of days free from self-isolation among DCT participants who reported LFD tests was estimated as 121 115 days (mean of 5·4 days per participant [SD 1·72]).
Regarding PCR testing uptake and compliance, 66 536 valid PCR results with specimen dates 2 days before recruitment to 14 days after recruitment (to cover a full incubation period) were obtained from 38 080 participants using England's national laboratory surveillance system; 17 387 (74·0%) of 23 500 self-isolation participants and 20 693 (79·2%) of 20 693 DCT participants (table 4 ). 810 (1·2%) results were void. The median number of PCR tests taken by participants during this time period was two for both groups (self-isolation: IQR 0–2, range 0–9; DCT: IQR 1–2, range 0–8), with 12 061 (68·5%) DCT participants who submitted an LFD result to the study portal submitting two or more PCR swabs in the 14 days following recruitment, as directed by the study protocol. Of 17 614 DCT participants who submitted an LFD result to the study portal and submitted a PCR swab, 1647 (9·4%) had a positive PCR result.
Table 4.
PCR results by test and by participant
| Self-isolation group | DCT group | Total | |
|---|---|---|---|
| Results by test | |||
| Negative | 23 434 | 36 518 | 59 952 |
| Positive | 2797 | 2977 | 5774 |
| Void | 317 | 493 | 810 |
| Total | 26 548 | 39 988 | 66 536 |
| Results by participant | |||
| Negative or void only | 15 023 | 18 363 | 33 386 |
| Positive | 2364 | 2330 | 4694 |
| Total | 17 387 | 20 693 | 38 080 |
| Proportion positive | 13·6% | 11·3% | 12·3% |
DCT=daily contact testing.
31 660 (63%) participants responded to the baseline questionnaire; 17 694 in the self-isolation and 13 966 in the DCT group. 69% of all respondents reported participating in the study as they wanted to avoid self-isolating if possible (appendix p 23).
20 004 (40%) participants responded to the end-of-study questionnaire (8807 self-isolation-participants, 754 in the DCT positive-test subgroup and 10 443 in the DCT no-positive-test subgroup; table 5 ). 82% of individuals in the self-isolation group reported much less contact with non-household contacts in the previous 7 days compared with the week before, as did 84% of individuals in the DCT positive-test subgroup. In the DCT no-positive-test subgroup, 57% reported much less contact, with 11% of participants reporting having much or slightly more contact. Participants were asked about any reasons for leaving home while self-isolating. The most common response was to take a COVID-19 test (87% of those who reported a reason in both groups). The proportion of participants who reported at least one other activity outside of the home while self-isolating was similar between the self-isolation group and DCT positive-test subgroup (16% [n=1409] and 17% [n=128], respectively; p=0·80). In the self-isolation group, most respondents (79%) were very or completely confident in the accuracy of their test results. This level of confidence was reported by 64% of participants in the DCT positive-test subgroup and 83% in the DCT no-positive-test subgroup.
Table 5.
Final survey behavioural responses, by group
| Self-isolation group (n=8807) | DCT positive-test subgroup (n=754) | DCT no-positive-test subgroup (n=10 443) | p value* | |
|---|---|---|---|---|
| (1) Last 24 h activities† | ||||
| Work, college, university | 72 (25%) | 24 (20%) | 2462 (29%) | 0·028 |
| Other indoor place | 105 (37%) | 42 (35%) | 3813 (45%) | 0·0013 |
| Outdoors with friends or family | 72 (25%) | 21 (17%) | 2349 (28%) | 0·023 |
| Indoors with friends or family | 54 (19%) | 9 (7%) | 1146 (14%) | 0·0050 |
| Any other reason | 110 (39%) | 67 (55%) | 2984 (35%) | <0·0001 |
| None of these† | 487 (63%) | 631 (84%) | 2029 (20%) | <0·0001 |
| (2) 7 days close contacts during study period | ||||
| Much more | 209 (2%) | 9 (1%) | 374 (4%) | <0·0001 |
| Slightly more | 289 (3%) | 20 (3%) | 742 (7%) | .. |
| About the same | 699 (8%) | 67 (9%) | 1801 (17%) | .. |
| Slightly less | 415 (5%) | 28 (4%) | 1563 (15%) | .. |
| Much less | 7180 (82%) | 626 (84%) | 5946 (57%) | .. |
| (3) Confidence in test accuracy | ||||
| Completely | 3590 (43%) | 255 (40%) | 4638 (44%) | <0·0001 |
| Very | 2997 (36%) | 156 (24%) | 4025 (39%) | .. |
| Fairly | 1510 (18%) | 121 (19%) | 1617 (16%) | .. |
| Not very | 180 (2%) | 68 (11%) | 127 (1%) | .. |
| Not at all | 69 (1%) | 44 (7%) | 33 (0%) | .. |
| (4) Activities while self-isolating | ||||
| Work, college, university | 713 (19%) | 29 (13%) | .. | 0·055 |
| Other indoor place | 1264 (33%) | 107 (48%) | .. | <0·0001 |
| Outdoors with friends or family | 918 (24%) | 49 (22%) | .. | 0·64 |
| Indoors with friends or family | 684 (18%) | 29 (13%) | .. | 0·096 |
| Left home for any other reason | 3122 (81%) | 168 (76%) | .. | 0·088 |
| (5) Reasons for leaving home when isolating‡ | ||||
| Earn money | 74 (2%) | 4 (2%) | .. | 0·99 |
| Keep my job | 59 (1%) | 4 (2%) | .. | 0·61 |
| Practical reasons (shopping, caring, etc) | 393 (8%) | 13 (6%) | .. | 0·33 |
| Mental health | 668 (14%) | 15 (7%) | .. | 0·0054 |
| Other important things | 265 (6%) | 18 (9%) | .. | 0·100 |
| Probably not infectious | 152 (3%) | 8 (4%) | .. | 0·79 |
| Didn't leave home‡ | 4099 (47%) | 532 (73%) | .. | <0·0001 |
| Take a coronavirus test | 4084 (87%) | 182 (87%) | .. | 1·000 |
Data completeness for the self-isolation group, the DCT positive-test subgroup, and—for questions (1) to (3)—the DCT no-positive-test subgroup, respectively, is given for each question, as follows: (1) “Thinking about yesterday, please tick all the things you did”. Data completeness: 769/8807 (8·7%), 752/754 (99·7%), and 10 431/10 443 (99·9%) provided at least one response for items 1–6. (2) “Compared to the week before, in the last 7 days did you have more or less close contact with people you don't live with, indoors and for more than 15 minutes?” Data completeness: 8792 (99·8%), 750 (99·5%), and 10 426 (99·8%) responded to this question. (3) [If yes to: “In the past 7 days, did you take any tests for coronavirus?”] “How confident are you that your test results were accurate?” Data completeness: 8346 (94·8%), 644 (85·4%), and 10 440 (100·0%) responded to this question. (4) “Thinking about the last 7 days, how often have you done each of these things [since getting your first positive test result]?”. (Note: these were recoded as binary never vs once or more; options were: never, once or twice, three or four times, five times or more.) Data completeness: 3858 (43·8%) and 221 (29·3%) provided at least one response to items 1–5. (5) “Did you leave home [following a positive test result or during your self-isolation period] for any of the following reasons? (Please tick all that apply)”. Data completeness: 8678 (98·5%) and 733 (97·2%) provided at least one response to items 1–8. Except where noted, percentages are calculated from those who responded to each question. DCT=daily contact testing.
For items where respondents were asked to tick all responses that applied to them, p values were calculated for each 2 × 2 comparison. For items where respondents were asked to select a single response, an overall p value was calculated for that item.
Percentages for responses 1–5 are calculated from those who reported leaving home; for response 6 (none of these), the proportion is calculated among those who responded to any of items 1–6.
Percentages for responses 1–6 are calculated from those who reported at least one reason for leaving home, which includes people who left home to take a coronavirus test (data not presented here); for response 7 (I didn't leave home) the proportion is calculated among those who provided at least one reponse to this question.
Discussion
As the COVID-19 pandemic continues to have a major impact on health and society, it is important to make efforts to reduce transmission. Although self-isolation of confirmed cases and their contacts can be effective, it is also disruptive for society and causes adverse impacts for individuals, given the practical, financial, and psychological challenges associated with sustained and repeated self-isolation. Our study was completed before the removal of all social restrictions in England. At the time, data were needed to develop innovative approaches to limit self-isolation to those infected with SARS-CoV-2, while allowing those without infection to return to greater normality.14, 20 Although previous studies had shown that DCT was acceptable to participants,14, 15, 16 these studies were not powered to assess the potential transmission risk to others.
Here we present results of the first randomised controlled trial in a general population of close contacts of COVID-19 cases comparing standard self-isolation with DCT (with 24 h exemption from self-isolation after a negative LFD result). By ascertaining the proportion of contacts of PCR-positive study participants who became tertiary cases in each group, we demonstrated that DCT was a safe alternative to self-isolation regarding onwards transmission of SARS-CoV-2, with a difference in attack rates of –1·2% among contacts of secondary cases, indicating non-inferiority of DCT. Consistent with this finding, the number of observed tertiary cases per secondary case was 0·14 in the DCT group and 0·16 in the self-isolation group. Attack rates among contacts of DCT participants who had received two vaccine doses (>14 days before recruitment) also showed non-inferiority compared with self-isolation participants, with an adjusted difference between DCT and self-isolation groups (self-isolation as baseline) of –1·6%. This finding suggests that DCT would be of value among fully vaccinated people. Although there was some imbalance in the study groups due to household clustering, a sensitivity analysis restricted to the first person recruited also showed that DCT was non-inferior to self-isolation. Other sensitivity analyses adjusting for non-reporting of LFD results supported the plausibility of DCT non-inferiority.
Although our study did not set out to change behaviour, individuals in the DCT group who reported only negative test results did not report significantly more contacts than those who were self-isolating, with the majority of contacts reported by participants in both groups being household contacts. It is possible that DCT might perform differently in the absence of social restrictions, when cases are likely to have a higher number of close contacts beyond the household.
Compliance with testing was high in our study, with around 80% of DCT participants submitting at least one LFD result, higher than the 70·2% observed in the pilot study.14 Furthermore, 74·0% of self-isolation participants and 79·2% of DCT participants had at least one PCR test during the study, higher than the 40% return rate reported in a previous study.13
DCT participants living in more socioeconomically deprived areas and those from minority ethnic backgrounds were less likely to report an LFD result, aligning with previous findings.14, 16 The ineligibility of DCT participants for isolation support payments might have resulted in lower compliance among participants in lower socioeconomic groups.4 Before introducing any DCT policy, it will be important to engage with disadvantaged communities to further understand and address barriers to testing and reporting.
The average number of close contacts reported was low (2·7 contacts per case), consistent with the national experience.25 Around 80% of secondary cases (participants who tested positive by PCR) provided details of their contacts to NHSTT, which was similar to figures for overall compliance with the contact tracing programme.25 During the study period, 30–50% of contacts notified to NHSTT in England were non-household contacts.25 This figure was substantially higher than the proportion of non-household contacts reported in the study for both groups (around 12%), with the number of non-household contacts per case nearly equivalent between groups. This lower number of non-household contacts might indicate increased caution, as described in a qualitative analysis,19 or ascertainment bias towards being early in the isolation period. Study participants were aware of their contact status before becoming cases and therefore might have had different behaviours to the general case population, which includes cases who were not previously aware of their contact status. Although additional freedom was offered to DCT participants, individuals in the DCT group were still advised to limit contact and that they should engage only in essential activities. Behavioural data suggest that DCT participants limited their contact with others and remained cautious in their behaviours despite enjoying additional freedom.19 For example, 57% of DCT participants reported having much less contact with people they did not live with in the last 7 days, compared with the week before, with only 11% of DCT participants reporting having much or slightly more contact. By contrast, around 80% of participants in both the self-isolation group and the DCT positive-test subgroup reported much less contact with non-household contacts, with compliance with self-isolation similar to estimates from a study conducted by the Office for National Statistics.5
The study was undertaken as part of real-life management of contacts of COVID-19 cases, providing evidence of the impact that a DCT policy would have, if introduced. More than 2 years since the identification of SARS-CoV-2, the pandemic continues to have a substantial impact on individuals and society. Developing public health interventions that mitigate both viral transmission and the wider impacts on health, wellbeing, prosperity, and society, including those arising from self-isolation, is essential. This study shows the potential benefits of daily testing while minimising the need for self-isolation.
The legal duty to self-isolate if testing positive for COVID-19 was removed in England from Feb 24, 2022,26 and widespread public access to free PCR and LFD tests was removed on April 1, 2022, although free asymptomatic testing remained in place for some settings and groups until removed on Aug 31, 2022.27 Public health guidance has remained in place during this time,28 encouraging those with symptoms or who have tested positive for COVID-19 using a test they have paid for to minimise contact with others, stay at home and work from home if possible, and exercise judgement about their personal risk and risk to others, particularly vulnerable individuals. Although testing rates and adherence to self-isolation substantially reduced in the weeks following the removal of the legal duty,29 a sizeable minority appear still to be taking steps to reduce the risks of COVID-19 transmission.30 LFD tests remain a valuable way that individuals can assess their infection status, and paid-for LFD tests are widely available. Although current public health guidance does not include the recommendation for individuals to test daily, the data presented in this paper support DCT as a viable strategy for future pandemic scenarios.
The key strengths of this RCT include its large size, the real-life setting using existing contact tracing systems, and validity of the transmission measure due to the use of named contacts identified by their exposers. There are, however, some limitations. We relied on self-notification of close contacts, which could lead to underascertainment, particularly of non-household contacts. These limitations are also limitations of the existing contact tracing system. Furthermore, it was not possible to assess the risk of transmission beyond named close contacts. The attack rates should be considered minimum estimates because only contacts who access testing can subsequently be identified as a case and because, to avoid mismatching, the process to identify transmission was highly specific. Despite freedoms allowed, DCT participants were still advised to minimise contact and national restrictions were in place at the time of our study, which might have reduced likelihood of onward transmission. There was a skew in the DCT group towards individuals who were able to work from home, which again would limit opportunities for non-household transmission. Differences in the number of contacts within workplaces and non-household settings could not be fully explored in this work because of national COVID-19 restrictions in place during the study period. Findings might not be generalisable to children; however, other work has investigated DCT in schools.18
The COVID-19 pandemic has accelerated innovation in infectious diseases diagnostics, with an expansion of self-testing and high-volume rapid asymptomatic testing with the aim of reducing transmission. These approaches to testing could, in future, enable more dynamic monitoring of transmission patterns. Community engagement and participation in self-testing has been variable. Therefore, there is a need to develop rapid evaluation approaches that are co-produced with communities to ensure effective testing policies in future pandemics and epidemics.
Data sharing
Deidentified testing data are available with an associated data dictionary. Access to data will be determined by the chief investigator (IO) after review of the proposal with a signed data access agreement. Data should be requested by contacting the corresponding author.
Declaration of interests
IO, GJR, and LY participate in the UK Scientific Advisory Group for Emergencies and its subgroups, where this and related work were discussed. CT also contributed to these groups, presenting analysis as part of work on the COVID response. GJR has been paid by Bayer as a legal expert witness, not related to this manuscript. GJR received payment for peer review of research grants and manuscripts related to COVID-19, but not related to this manuscript. Several authors (IO, SH, NL, DR, AFM, SD, LY, GJR, RA, and CT) carried out previous studies funded by the Department of Health and Social Care investigating testing of contacts of COVID-19 cases by PCR or LFD. Several authors (IO, LY, and JR) were funded by UK Research and Innovation or the National Institute for Health and Care Research (NIHR) to undertake research related to COVID-19, outside of the current study. IO, DR, CEF, IO, RA, SD, and LY receive funding from the NIHR Health Protection Research Unit (HPRU) in Behavioural Science and Evaluation at the University of Bristol. NL receives funding from the NIHR HPRU in Gastrointestinal Infections at the University of Liverpool. LY is an NIHR Senior Investigator, and her research programme is partly supported by NIHR Applied Research Collaboration West and NIHR Biomedical Research Centre at the University of Southampton. RA and GJR receive payments from the NIHR HPRU in Emergency Preparedness and Response. LY, SD, and IO receive payments from the Department of Health and Social Care. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
Funding was provided by the UK Government Department of Health and Social Care. AFM is supported by the Economic and Social Research Council (grant number ES/J500057/1). We would like to thank the following teams for supporting the delivery of the trial: logistical and operational support from the NHS Test & Trace daily contact testing and home delivery teams (UKHSA and Department of Health and Social Care); recruitment support from the Agile Lighthouse team (UKHSA), NHSTT call handlers, and NHS 119 call handlers; administrative and mailbox support from the contact tracing administration team (UKHSA); data linkage by the Second Generation Surveillance System team and Field Service South West (UKHSA); and data support and advice from EpiCell and Contact tracing teams (UKHSA).
Contributors
NKL, DRR, CT, SH, and IO conceptualised the study. NKL performed data curation and NKL and SD provided resources for the study. NKL, CT, NQV, CEF, and AFM undertook formal data analysis. TBS and SM performed data validation. NKL, CT, CEF, and AFM performed visualisation. NKL and DRR were involved in the investigation. NKL, DRR, CT, NQV, CEF, SD, GJR, LY, RA, SH, and IO contributed to the methodology. NKL, DRR, and CEF performed project administration. DRR and IO undertook supervision. NKL, DRR, CT, and IO wrote the original draft and all authors contributed to the review and editing of the manuscript. NKL, DRR, CT, NQV, TBS, SM, SH, and IO had full access to all the data in the study. NKL, CT, NVQ, TBS, and SM accessed and verified the data. All authors had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Department for Health and Social Care NHS Test and Trace: how it works. https://www.gov.uk/guidance/nhs-test-and-trace-how-it-works
- 2.UK Health Security Agency Guidance for contacts of people with confirmed coronavirus (COVID-19) infection who do not live with the person. https://www.gov.uk/government/publications/guidance-for-contacts-of-people-with-possible-or-confirmed-coronavirus-covid-19-infection-who-do-not-live-with-the-person
- 3.Denford S, Morton KS, Lambert H, et al. Understanding patterns of adherence to COVID-19 mitigation measures: a qualitative interview study. J Public Health (Oxf) 2021;43:508–516. doi: 10.1093/pubmed/fdab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith LE, Aml-t R, Lambert H, et al. Factors associated with adherence to self-isolation and lockdown measures in the UK: a cross-sectional survey. Public Health. 2020;187:41–52. doi: 10.1016/j.puhe.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Office for National Statistics Coronavirus and self-isolation after being in contact with a positive case in England: 9 to 16 August 2021. Sept 8, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronavirusandselfisolationafterbeingincontactwithapositivecaseinengland/9to16august2021
- 6.Smith LE, Potts HWW, Aml-t R, Fear NT, Michie S, Rubin GJ. Adherence to the test, trace and isolate system: results from a time series of 21 nationally representative surveys in the UK (the COVID-19 Rapid Survey of Adherence to Interventions and Responses [CORSAIR] study) 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/928684/S0732_CORSAIR_-_Adherence_to_the_test__trace_and_isolate_system.pdf
- 7.Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quilty BJ, Clifford S, Hellewell J, et al. Quarantine and testing strategies in contact tracing for SARS-CoV-2: a modelling study. Lancet Public Health. 2021;6:e175–e183. doi: 10.1016/S2468-2667(20)30308-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity—a strategy for containment. N Engl J Med. 2020;383:e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 10.Mina MJ, Andersen KG. COVID-19 testing: one size does not fit all. Science. 2021;371:126–127. doi: 10.1126/science.abe9187. [DOI] [PubMed] [Google Scholar]
- 11.UK Government Department of Health and Social Care Understanding lateral flow testing for people without symptoms. https://www.gov.uk/guidance/understanding-lateral-flow-antigen-testing-for-people-without-symptoms
- 12.Peto T, UK COVID-19 Lateral Flow Oversight Team COVID-19: rapid antigen detection for SARS-CoV-2 by lateral flow assay: a national systematic evaluation of sensitivity and specificity for mass-testing. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchant E, Ready D, Wimbury G, Smithson R, Charlett A, Oliver I. Determining the acceptability of testing contacts of confirmed COVID-19 cases to improve secondary case ascertainment. J Public Health (Oxf) 2021;43:e446–e452. doi: 10.1093/pubmed/fdab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Love N, Ready D, Turner C, et al. The acceptability of testing contacts of confirmed COVID-19 cases using serial, self-administered lateral flow devices as an alternative to self-isolation. J Med Microbiol. 2022;71 doi: 10.1099/jmm.0.001567. [DOI] [PubMed] [Google Scholar]
- 15.Denford S, Martin AF, Love N, et al. Engagement with daily testing instead of self-isolating in contacts of confirmed cases of SARS-CoV-2: a qualitative analysis. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.714041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin AF, Denford S, Love N, et al. Engagement with daily testing instead of quarantine following possible exposure to SARS-CoV-2. BMC Public Health. 2021;21 doi: 10.1186/s12889-021-11135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quilty BJ, Clifford S, Hellewell J, et al. Quarantine and testing strategies in contact tracing for SARS-CoV-2: a modelling study. Lancet Public Health. 2021;6:e175–e183. doi: 10.1016/S2468-2667(20)30308-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young BC, Eyre DW, Kendrick S, et al. Daily testing for contacts of individuals with SARS-CoV-2 infection and attendance and SARS-CoV-2 transmission in English secondary schools and colleges: an open-label, cluster-randomised trial. Lancet. 2021;398:1217–1229. doi: 10.1016/S0140-6736(21)01908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denford S, Martin AF, Towler L, et al. A qualitative process analysis of daily contact testing as an alternative to self-isolation following close contact with a confirmed carrier of SARS-CoV-2. BMC Public Health. 2022;22 doi: 10.1186/s12889-022-13800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Department for Health and Social Care Asymptomatic testing for SARSCoV-2 using antigen-detecting lateral flow devices. Asymptomatic testing for SARS-CoV-2 using antigen-detecting lateral flow devices: evidence from performance data October 2020 – May 2021. July 7, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/999866/asymptomatic-testing-for-SARS-CoV-2-using-antigen-detecting-lateral-flow-devices-evidence-from-performance-data-Oct-2020-to-May-2021.pdf
- 21.Dinnes J, Deeks JJ, Berhane S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3 doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royston P, Babiker A. A menu-driven facility for complex sample size calculation in randomized controlled trials with a survival or a binary outcome. Stata J. 2002;2:151–163. [Google Scholar]
- 23.Mansournia MA, Nazemipour M, Naimi AI, et al. Reflection on modern methods: demystifying robust standard errors for epidemiologists. Int J Epidemiol. 2021;50:346–351. doi: 10.1093/ije/dyaa260. [DOI] [PubMed] [Google Scholar]
- 24.Research Registry Registration details: 6809: Determining the risk of onward infection transmission from contacts of confirmed COVID-19 cases who are using serial, self-administered Lateral Flow Devices with a ‘test to enable’ approach or undertaking PCR with isolation: a randomised controlled trial. https://www.researchregistry.com/browse-the-registry#home/registrationdetails/609baef0a38915001cbdf123/
- 25.UK Health Security Agency Weekly statistics for NHS Test and Trace (England): 14 October to 20 October 2021. Oct 28, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1029536/test-and-trace-oct-14-to-20.pdf
- 26.UK Government Prime Minister's Office Oral statement to Parliament: PM statement on living with COVID. 21 February 2022. https://www.gov.uk/government/speeches/pm-statement-on-living-with-covid-21-february-2022
- 27.Department of Health and Social Care Regular asymptomatic testing paused in additional settings. https://www.gov.uk/government/news/regular-asymptomatic-testing-paused-in-additional-settings
- 28.UK Health Security Agency People with symptoms of a respiratory infection including COVID-19. https://www.gov.uk/guidance/people-with-symptoms-of-a-respiratory-infection-including-covid-19
- 29.Office for National Statistics Coronavirus and self-isolation after testing positive in England: 28 March to 2 April 2022. May 10, 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandwellbeing/bulletins/coronavirusandselfisolationaftertestingpositiveinengland/28marchto4april2022
- 30.Office for National Statistics Coronavirus (COVID-19) latest insights: lifestyle. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/lifestyle
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified testing data are available with an associated data dictionary. Access to data will be determined by the chief investigator (IO) after review of the proposal with a signed data access agreement. Data should be requested by contacting the corresponding author.