Figure 1. A chemical screen identifies etiocholanolone, a testosterone catabolite, as a pyrin inflammasome step 2 activator.
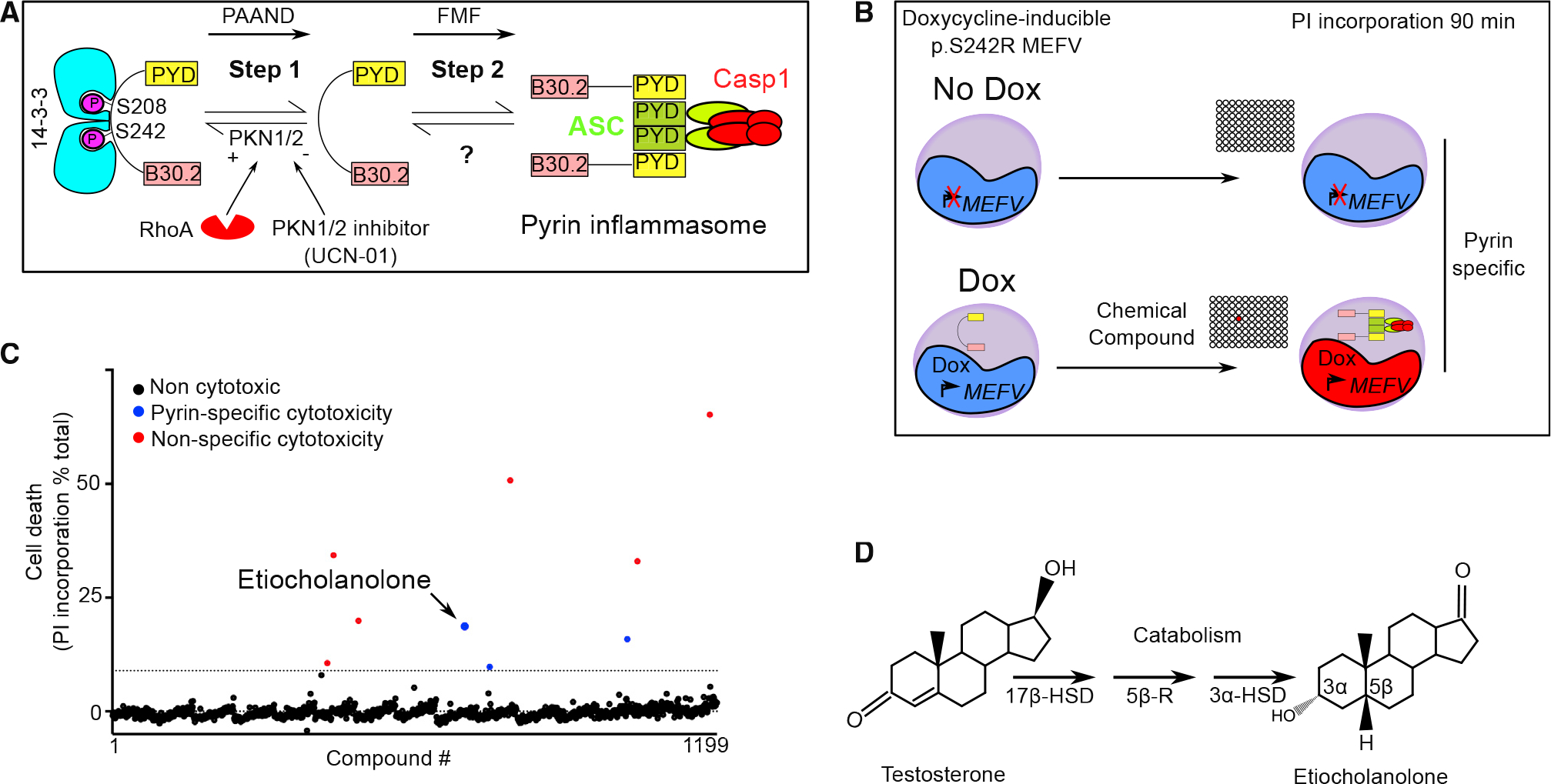
(A) Model for pyrin two-step activation mechanism. Step 1 is due to dephosphorylation of pyrin and loss of 14–3-3 binding and is constitutive in PAAND patients. Step 2 is uncharacterized but is upstream of ASC speck formation and is constitutive in FMF patients.
(B) Chemical screen overview: cells expressing doxycycline (Dox) or not (No Dox) p.S242R MEFV were exposed to individual chemical compounds. Ninety-minute post-exposure, cell death was monitored using propidium iodide (PI). Compounds driving cell death independently of pyrin (i.e., in the absence of Dox) were excluded.
(C) Screen results are shown, each dot represents the cell death value of cells exposed to one chemical compound. The dotted line represents the mean + 3 SD. Red dots represent non-specific hits (killing cells irrespective of the presence or the absence of Dox) while blue dots represent specific hits displaying cytotoxicity only upon pyrin expression. Etiocholanolone (Etio) (6.9 μM) is highlighted. Each chemical compound was screened once in the presence and in the absence of Dox. The value shown corresponds to normalized cell death value of a single well.
(D) Structure of progesterone and its catabolite etiocholanolone are shown. The stereochemistry of carbon 3 and 5 is indicated. 17β-HSD, 17β-hydroxy-steroid dehydrogenase; 5β-R, 5β-reductase; 3α-HSD, 3α-hydroxy-steroid dehydrogenase.
