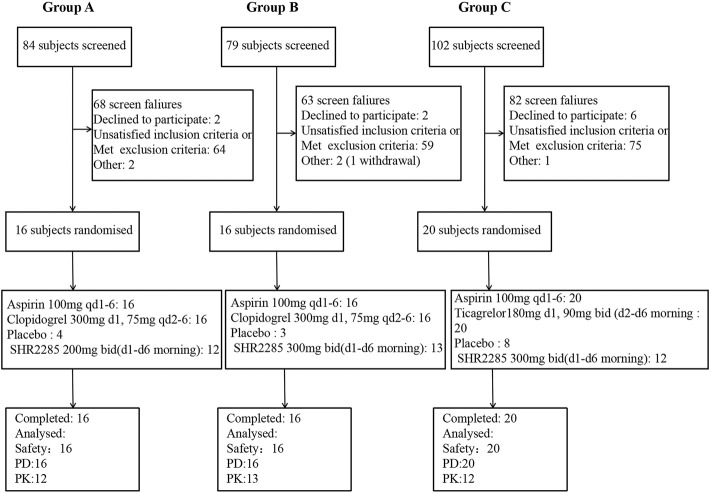
FIGURE 1.
(A) Subject disposition of participants in group A of the study (B) Subject disposition of participants in group B of the study. (C) Subject disposition of participants in group C of the study. PD, pharmacodynamics; PK, pharmacokinetics; qd, once daily; bid, twice a day, once in the morning and once in the evening. There was unwilling to see an error in the randomization system, resulting in three placebos and 13 trial drugs in group (B) (One subject withdrew due to an adverse event, and the substitute subject accepted the drug randomization).