Abstract
Background
Catheter ablation (CA) is an effective treatment for patients with persistent atrial fibrillation (PsAF); however, little is known about its impact on health care utilization for patients with PsAF. The ThermoCool SmartTouch SF (STSF) catheter (Biosense Webster) incorporates an advanced porous tip and contact force–sensing technology.
Objective
The purpose of this study was to determine health care utilization among patients with PsAF who underwent ablation with the STSF catheter.
Methods
A retrospective cohort study using the Premier Healthcare Database identified patients with PsAF undergoing CA with the STSF catheter in inpatient and outpatient settings. The proportion of patients experiencing AF-related inpatient admissions, outpatient admissions, emergency department (ED) visits, electrical cardioversion, and a composite outcome in the 12 months pre- vs postablation were compared using the McNemar test. Subanalyses were performed on study outcomes by race/ethnicity.
Results
The final sample included 3077 patients (mean age 65.9 years; 31.7% female). Among patients with PsAF undergoing ablation with the STSF catheter, relative reductions in health care utilization in the 12 months post- vs preablation included 55.3% in AF-related inpatient admissions (P <.0001), 38.9% in outpatient admissions (P <.0001), 52.4% in ED visits (P <.0001), and 61.2% in electrical cardioversions (P <.0001). Composite outcome utilization in the 12 months post- vs preablation declined by 40.2% (P <.0001) for the overall cohort, 40.0% for White patients (P <.0001), 52.2% for Black patients (P <.0001), and 50.1% for Asian patients (P = .032).
Conclusion
Significant improvements in health care utilization were observed among PsAF patients who underwent ablation using the STSF catheter. Improvements were particularly marked in underrepresented racial and ethnic groups.
Keywords: Atrial fibrillation, Catheter ablation, Contact force, Health care utilization, Race
Key Findings.
-
▪
In this real-world study using a nationwide, multihospital database, use of the ThermoCool SmartTouch SF (STSF) catheter during a catheter ablation procedure for patients with persistent atrial fibrillation (PsAF) led to significant reductions in health care utilization.
-
▪
Among patients with PsAF undergoing ablation with the STSF catheter, relative reductions in use in the 12-month post- vs preablation period included declines >50% in atrial fibrillation (AF)–related inpatient admissions (P <.0001), emergency department visits (P <.0001), and electrical cardioversions (P <.0001).
-
▪
Use of the STSF device for PsAF treatment led to meaningful improvements in health care utilization across different racial and ethnic groups. The improvements in a composite outcome, which included AF-related inpatient, outpatient, and emergency department admissions as well as cardioversions, were particularly pronounced among Black and Asian patients.
Introduction
Atrial fibrillation (AF) currently affects approximately 5 million Americans and is expected to affect as many as 12.1 million people by 2030.1 Considerable health care burden is associated with AF. Patients with AF are 3 times more likely to be hospitalized compared to those without AF.2 Health care cost among individuals with AF has been shown to be ∼$8705 more per individual per year compared to those without AF.2 The total economic burden of AF in the United States (US) is around $30 billion, and this figure is expected to increase to $65.7 billion by 2035.3
Randomized controlled trials have demonstrated that catheter ablation (CA) reduces long-term AF recurrence in patients with drug-refractory paroxysmal AF and as first-line therapy.4,5 Similar results have been observed for patients with persistent atrial fibrillation (PsAF).6, 7, 8 In a randomized controlled trial comparing CA with antiarrhythmic drugs (AADs) among PsAF patients, the proportion of patients free of any recurrence was higher in the ablation arm compared to the AAD arm (60.2% vs 29.2%; P <.001).7 Recent developments in radiofrequency ablation are likely to further improve procedural outcomes.9 Conventional catheters provide only limited information on lesion formation. In contrast, catheter-to-tissue contact force (CF)–sensing technology confirms appropriate pressure by the ablation catheter to effectively facilitate radiofrequency energy transfer to the atrial myocardium.10 This information on real-time catheter-to-tissue interaction improves the ability to create durable lesions. In addition, optimizing catheter tip irrigation can result in more homogeneous cooling of the catheter tip and allow for the delivery of greater quantities of radiofrequency power by reducing the risk of overheating, which may result in larger lesions with greater depth.9 The ThermoCool SmartTouch SF (STSF) catheter (Biosense Webster, Irvine, CA) incorporates both CF-sensing technology and advanced 56-hole porous tip irrigation.
Studies have found that CA is cost-effective among patients with AF.11 Recent research indicates that these health care utilization improvements may also be realized among patients with PsAF.12 In an analysis of US administrative claims database, Friedman et al12 found that among patients with PsAF, CA was associated with a 64% reduction in inpatient admission (21.5% vs 7.8%; P <.0001), 59% reduction in emergency department (ED) visits (15.7% vs 6.4%; P <.0001), and 54% reduction in cardioversion (56.8% vs 26.4%; P <.0001) in the 12-month post- vs preablation period.12 These reductions in medical services utilization postablation were observed to translate into health care cost savings, with AF-related inpatient admission costs declining by 33% (P <.0001), ED visit costs declining by 70% (P <.0001), and cardioversion costs declining by 55% (P <.0001) in the 12-month post- vs preablation period.
Given the expected growth in the number of individuals with AF combined with the high disease burden imposed by AF, the effect of CA on health care utilization has considerable implications for patients, payors, and providers. The primary objective of this study was to examine changes in health care utilization in the 12-month pre- vs 12-month postablation period among patients with PsAF who underwent CA with the STSF catheter.
Methods
Data source
This was a retrospective cohort study using the 2017–2020 Premier Healthcare Database (PHD). The PHD is a nationwide, hospital billing database that includes complete clinical coding, hospital cost, and billing data from more than 1000 hospitals in the United States. Federally funded hospitals (eg, Veterans Affairs) are excluded from PHD; however, the hospitals that are included are representative of different bed size, geographic region, location (urban/rural), and teaching status. PHD is an aggregated, deidentified dataset in which no one patient can be individually identified. Therefore, patient consent is not required, and the study is Institutional Review Board exempt. The research reported in this paper adhered to guidelines set forth by the Helsinki Declaration as revised in 2013.
Study sample
The study sample included adult patients (≥18 years old) with a primary diagnosis of PsAF (ICD-10-CM I48.1) who underwent a CA procedure in an inpatient or outpatient setting using the STSF catheter between January 2017 and December 2019. The first CA procedure meeting these criteria was designated as the index ablation. Patients were required to have index CA in a hospital that continuously provided data to the PHD for the 12-month pre- and postindex ablation periods. Patients were excluded if they previously had undergone catheter or surgical ablation, a valvular procedure, or left atrial appendage occlusion in the 12-month preindex ablation period.
Patient demographics (age, gender, race, marital status), payor information, and major comorbidities (sleep apnea, dyslipidemia, cardiomyopathy) were collected. The CHA2DS2-VASc score, which calculates stroke risk for patients with AF,13,14 and the Elixhauser Comorbidity Index, an aggregate measure of comorbidity created using select diagnoses associated with 31 categories,15 were assessed, as were provider characteristics including region, teaching status, and bed size.
Outcome measures
Primary outcome measures included AF-related inpatient readmissions, AF-related outpatient readmissions, AF-related ED visits, direct current cardioversion (DCCV), and a composite endpoint including all 4 prior outcomes, assessed in the 12-month pre- and postindex CA periods. Sensitivity analyses were performed by examining study outcomes among a cohort of patients who did not undergo repeat ablation in the 12-month period postindex ablation. To better understand the impact on health care utilization among underrepresented racial and ethnic groups, we performed subanalyses by examining study outcomes among different race/ethnic groups (specifically White, Black, and Asian patients).
Data analysis
The McNemar test was used to compare changes in the proportion of patients experiencing study outcomes in the pre- and postindex CA periods.16 In all analyses, 2-sided P <.05 was the threshold by which differences were considered significant. All analyses were conducted using R Studio.
Results
There were 3077 patients who met the study criteria (Supplemental Figure 1). Mean (± SD) age of the final sample was 65.9 ± 9.5 years, and 31.7% were female. Mean Elixhauser score was 3.5 ± 1.7, and mean CHA₂DS₂-VASc score was 2.6 ± 1.5. About one-third of patients had sleep apnea, 20.9% had cardiomyopathy, and 51.2% had dyslipidemia. Around 80% of procedures were performed in an outpatient setting. Per provider characteristics, 64.8% were based in the South, 57.6% were teaching hospitals, and 67.0% had ≥500 beds. Table 1 lists the study patient characteristics.
Table 1.
Demographic, comorbid characteristics, and hospital characteristics for the study sample (N = 3077)
| Age (y) | 65.9 ± 9.5 |
| Age group (y) | |
| 18–44 | 158 (5.1) |
| 44–54 | 572 (18.6) |
| 55–64 | 1136 (36.9) |
| ≥65 | 1211 (39.3) |
| Gender | |
| Female | 976 (31.7) |
| Male | 2101 (68.3) |
| Race | |
| White | 2669 (86.7) |
| Black | 141 (4.6) |
| Asian | 28 (0.9) |
| Other | 132 (4.3) |
| Unknown | 107 (3.5) |
| Payor | |
| Medicare or Medicaid | 1935 (62.9) |
| Commercial | 1154 (37.5) |
| Other | 71 (2.3) |
| Elixhauser score | 3.5 ± 1.7 |
| CHA₂DS₂-VASc score | 2.6 ± 1.5 |
| Comorbidities | |
| Sleep apnea | 1038 (33.7) |
| Dyslipidemia | 1576 (51.2) |
| Cardiomyopathy | 643 (20.9) |
| Procedure setting | |
| Inpatient | 612 (19.9) |
| Outpatient | 2465 (80.1) |
| Hospital location | |
| South | 1993 (64.8) |
| Midwest | 622 (20.2) |
| Northeast | 405 (13.2) |
| West | 57 (1.9) |
| Hospital teaching status | |
| Yes | 1772 (57.6) |
| No | 1305 (42.4) |
| Hospital bed size | |
| ≥500 beds | 2,061 (67.0) |
| 300–499 beds | 692 (22.5) |
| 000–299 beds | 324 (10.5) |
Values are given as mean ± SD or n (%).
CHA₂DS₂-VASc = congestive heart failure, hypertension, age ≥75, diabetes, stroke, vascular disease, age 65 to 74, and sex category.
Significant improvements in the proportions of PsAF patients experiencing health care utilization were observed (Figure 1 and Table 2). In the 12-month postablation period compared to the 12-month preablation period, a 55.3% relative reduction in all-cause inpatient readmissions (P <.0001; absolute difference in proportions [ADP] –7.4%; 95% confidence interval [CI] –8.9% to –5.9%), 38.9% reduction in AF-related outpatient readmissions (P <.0001; ADP –21.4%; 95% CI –23.8% to –18.9%), and 52.4% reduction in AF-related ED visits (P <.0001; ADP –1.8%; 95% CI –2.6% to –0.9%) were observed among PsAF patients who had CA using the STSF catheter. DCCVs decreased by 61.2% (P <.0001; ADP –20.6%; 95% CI –22.7% to –18.5%) in the 12-month postablation period compared to the preablation period. Overall, a reduction of 40.2% (P <.0001; ADP –24.4%; 95% CI –26.9% to –22.0%) was observed in the composite endpoint in the 12-month post- vs preablation period. Results from sensitivity analysis aligned with those from main analyses, after excluding patients who had undergone repeat ablation in the 12-month postindex ablation period (5.82% [179] of patients had repeat ablation and were removed). In the sample for sensitivity analyses, AF-related inpatient admissions decreased by 65.1% (P <.0001), AF-related outpatient admissions decreased by 44.9% (P <.0001), AF-related ED visits decreased by 61.4% (P <.0001), DCCV decreased by 66.1% (P <.0001), and composite admissions decreased by 46.5% (P <.0001) in the 12-month postablation period compared to the preablation period (Supplemental Table 1 and Supplemental Figure 2).
Figure 1.
Relative percent change in the proportion of study outcomes in the 12-month pre- and postablation periods. AF = atrial fibrillation; DCCV = direct current cardioversion.
Table 2.
Absolute difference in the proportion of study outcomes in the 12-month pre- and postablation periods
| Admissions | Patients (N = 3077) |
Absolute difference in proportion (%) | |
|---|---|---|---|
| Pre 12 months | Post 12 months | ||
| AF-related inpatient | 412 (13.4) | 184 (6.0) | –7.4 (–8.9 to –5.9) |
| AF-related outpatient | 1690 (54.9) | 1033 (33.6) | –21.4 (–23.8 to –18.9) |
| AF-related ED | 103 (3.3) | 49 (1.6) | –1.8 (–2.6 to –0.9) |
| DCCV | 1035 (33.6) | 402 (13.1) | –20.6 (–22.7 to –18.5) |
| Composite | 1872 (60.8) | 1120 (36.4) | –24.4 (–26.9 to –22.0) |
Values are given as n (%) or 95% confidence interval.
AF = atrial fibrillation; DCCV = direct current cardioversion; ED = emergency department.
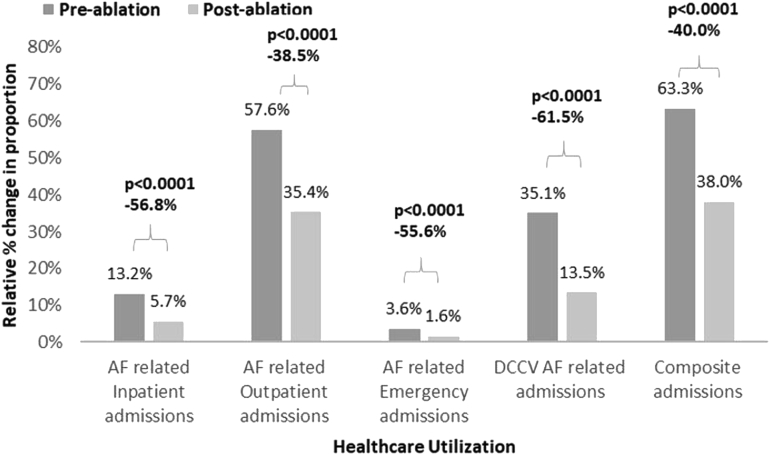
When examining study outcomes among White patients (Figure 2 and Table 3), a 56.8% relative decrease was observed in the proportion of patients experiencing AF-related inpatient admissions (P <.0001), 38.5% decrease in outpatient admissions (P <.0001), 55.6% decrease in ED visits (P <.0001), 61.5% decrease in DCCV (P <.0001), and 40.0% decrease in composite outcome (P <.0001) in the 12-month postablation period vs the preablation period. Among Black patients, the proportion of patients experiencing AF-related inpatient admission declined by 65.2% (P = .003), 54.3% for outpatient admissions (P <.0001), 80.5% for DCCV (P <.0001), and 52.2% for composite outcome (P <.0001) in the 12-month post- vs pre-CA period (Figure 3 and Table 3). In Asian patients, the proportion experiencing the composite outcome declined by 50.1% (P = .032) (Figure 4 and Table 3).
Figure 2.
Relative percent change in the proportion of study outcomes in the 12-month pre- and postablation periods among White patients. AF = atrial fibrillation; DCCV = direct current cardioversion.
Table 3.
Absolute difference in the proportion of study outcomes in the 12-month pre- and postablation periods by race
| Admissions | White (N = 2669) |
Black (N = 141) |
Asian (N = 28) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre 12 months | Post 12 months | Absolute difference in proportion (%) | Pre 12 months | Post 12 months | Absolute difference in proportion (%) | Pre 12 months | Post 12 months | Absolute difference in proportion (%) | |
| Inpatient∗ | 352 (13.2) | 151 (5.7) | –7.5 (–9.1 to –5.9) | 26 (18.4) | 9 (6.4) | –12.0 (–20.3 to –3.8) | 4 (14.3) | 2 (7.1) | –7.2 (–26.8 to 12.5) |
| Outpatient∗ | 1538 (57.6) | 945 (35.4) | –22.2 (–24.9 to –19.6) | 59 (41.8) | 27 (19.1) | –22.7 (–33.8 to –11.6) | 16 (57.1) | 9 (32.1) | –25.0 (–53.8 to 3.8) |
| ED∗ | 96 (3.6) | 42 (1.6) | –2.0 (–2.9 to –1.1) | 2 (1.4) | 2 (1.4) | –0.0 (–2.8 to 2.8) | 2 (7.1) | 1 (3.6) | –3.5 (–18.9 to 11.8) |
| DCCV | 938 (35.1) | 361 (13.5) | –21.6 (–23.9 to –19.4) | 31 (22.0) | 6 (4.3) | –17.7 (–26.0 to –9.4) | 7 (25.0) | 4 (14.3) | –10.7 (–34.9 to 13.5) |
| Composite | 1690 (63.3) | 1015 (38.0) | –25.3 (–27.9 to –22.7) | 71 (50.4) | 34 (24.1) | –26.3 (–37.8 to –14.7) | 18 (64.3) | 9 (32.1) | –32.2 (–60.5 to –3.8) |
Values are given as n (%) or 95% confidence interval.
Abbreviations as in Table 2.
Atrial fibrillation-related admissions.
Figure 3.
Relative percent change in the proportion of study outcomes in the 12-month pre- and postablation periods among Black patients. AF = atrial fibrillation; DCCV = direct current cardioversion
Figure 4.
Relative percent change in the proportion of study outcomes in the 12-month pre- and postablation periods among Asian patients. AF = atrial fibrillation; DCCV = direct current cardioversion.
Discussion
In this real-world study of a nationwide, multihospital database, use of the STSF catheter during a CA procedure for patients with PsAF led to significant reductions in health care utilization. A lower proportion of patients experienced AF-related medical services utilizations in the 12-month postablation period compared to the 12-month preablation period. To the best of our knowledge, this is the first study to describe the impact on health care utilization associated with the STSF use among patients with PsAF in a real-world setting.
In a randomized controlled trial comparing CA with AAD for the treatment of patients with symptomatic PsAF, treatment with CA was found to be superior, as defined by freedom from sustained episodes of AF (ie, episodes of AF or atrial flutter lasting >24 hours or requiring cardioversion), at 12-month follow-up.7 The study reported 70.4% of patients in the CA arm to have freedom from AF at 12 months vs 43.7% of patients in the AAD arm (P = .002), with an absolute risk difference of 26.6% (95% CI 10.0–43.3). As such, the use of CA, before the use of Class I or III AADs, as first-line therapy, is considered appropriate for patients with PsAF.17 Our study adds to the existing body of evidence demonstrating that the superior treatment efficacy of CA translates into lower health care utilization, including that among patients with PsAF. Using a similar study design, Ladapo et al18 found significant reductions in the number of outpatient appointments, inpatient days, and ED visits among continuously enrolled patients 6 months postablation compared to 6 months preablation. In a more recent study, Field et al19 examined reductions in health care utilization among patients with PsAF undergoing CA (device agnostic) in the 12-month pre- vs postablation period. The authors reported 64% reduction in AF-related inpatient admissions, 59% reduction in ED visits, and 13% reduction in cardioversions in the 12-month post- vs preablation period among PsAF patients. Our results align with those from Field et al, with significant reduction in health care utilization observed among patients with PsAF treated with STSF catheter in our study.
Our findings extend previous research that focused on PsAF patients undergoing ablation using the STSF catheter. As CA technology evolves, there are likely to be variations in success rates among ablation catheters, with the expectation that newer catheters will have improved outcomes compared to those created with earlier technologies.20 Recent research indicates that the technological improvements incorporated in the STSF design may translate into improved procedural success and clinical outcomes.9,21 Results from the PRECEPT (Prospective Review of the Safety and Effectiveness of the THERMOCOOL SMARTTOUCH SF Catheter Evaluated for Treating Symptomatic PersistenT AF) trial demonstrated a clinical success rate of 80.4% at 15 months for patients with PsAF treated with the STSF catheter.22 Afzal et al21 found that CF-sensing radiofrequency ablation catheters led to a 37% decrease in AF recurrence over a 12-month follow-up period compared to radiofrequency catheters without CF-sensing technology. Additionally, a recent study comparing safety and clinical outcomes among symptomatic, drug-refractory patients with paroxysmal AF or PsAF who underwent CA using the STSF catheter vs a historical cohort of patients who had ablation with the ThermoCool SmartTouch (ST) (Biosense Webster, Diamond Bar, CA) catheter reported a 51% reduction in fluid delivery with the STSF catheter vs the ST catheter, and the 12-month arrhythmia-free survival rates were 79.9% for the STSF cohort vs 66.7% for the ST cohort (P = .18).9 They also found that the improved tip irrigation led to a significant reduction in procedural fluid burden, suggesting improved outcomes over previous CA techniques. Our research suggests these procedural and clinical improvements do translate into meaningful reductions in health care utilization among PsAF patients, thereby alleviating disease burden on patients, payors, and providers.
Given the expected growth in AF incidence and prevalence with demographic changes,23 interventions that are associated with reductions in health care utilization could lower the financial burden associated with this chronic condition. In 2011, Ladapo et al18 found that the savings associated with CA at least partially offset the cost of CA. As payors and providers navigate an increasingly challenging economic and health care services arena, made even more so by the coronavirus disease 2019 (COVID-19) pandemic, outcomes improvements as observed in our study with the use of the STSF catheter for CA reflect the incremental gains that they may capture with the use of this advanced technology among PsAF patients. From the patient perspective, a reduced health care burden likely would translate into a better quality of life and reduction in disease burden.
Although the effectiveness of the STSF device in treating PsAF has been studied, there is little information on how the performance differs across different racial and ethnic groups. To address inequities in health care, it is critical to examine device performance across different racial and ethnic groups, especially focusing on underrepresented groups. Our results suggest that the use of the STSF device for PsAF treatment leads to meaningful improvements in health care utilization across different racial and ethnic groups. In fact, the improvements were observed to be more pronounced among Black and Asian patients (from the perspective of composite outcome). These results suggest that the STSF device could be an effective tool in alleviating the health care burden imposed by PsAF in these underserved groups.
Study limitations
Given the quasi-experimental pretest–posttest study design used in our study, the role of regression to the mean in explaining study results (ie, improvements in health care use in the postablation period compared to the preablation period) could not be ruled out. However, given the magnitude of reductions in health care use observed in the post- vs preablation period, it is unlikely that regression to the mean could itself explain these results. We had to rely on a combination of device name and/or catalog identifiers for identification of the STSF device in PHD. As such, we may have missed use of the STSF device among patients listed in the PHD. Until unique device identifiers are fully adopted, this approach, although not perfect, offers a feasible alternative to the assessment of device use in real-world datasets such as the PHD. Information on clinical elements including ablation approach, AAD use, and oral anticoagulant use, which potentially could influence outcomes that were assessed, were not available. In PHD, health care utilization can be assessed only if patients go to the same hospital. If a patient were to visit another hospital, irrespective of whether that other hospital contributes data to PHD, that visit would not be recorded. Thus, there could be an underestimation of health care utilization in PHD. Finally, any potential errors during billing coding could have influenced study results.
Conclusion
In this study of a large, multihospital, nationwide hospital dataset, use of the STSF catheter for ablation of PsAF led to significant reductions in health care utilization, including reductions in AF-related inpatient admissions, outpatient admissions, ED visits, and electrical cardioversions. These improvements were observed across different racial and ethnic groups and were particularly marked for patients from underrepresented racial and ethnic groups. As health care resources become constrained, reductions in health care utilization associated with use of the advanced STSF catheter for PsAF treatment could help alleviate the burden for patients, payors, and providers.
Acknowledgments
The authors acknowledge Superior Medical Experts for literature research and editorial assistance.
Acknowledgments
Funding Sources
This study was sponsored by Johnson and Johnson.
Disclosures
Sonia Maccioni and Dr Khanna are Johnson & Johnson employees. Sariki Meghana Preethi is an employee of MuSigma Inc., which has a contractual relationship with Johnson & Johnson. Dr Doshi has no conflicts of interest to declare.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
As the study used retrospective, de-identified, hospital billing data, patient consent was not required for study purposes.
Ethics Statement
The Premier Healthcare Database is an aggregated, deidentified dataset in which no one patient can be individually identified. The study is Institutional Review Board exempt. The research reported in this paper adhered to guidelines set forth by the Helsinki Declaration as revised in 2013.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2022.07.003.
Supplementary data
References
- 1.Colilla S., Crow A., Petkun W., Singer D.E., Simon T., Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Kim M.H., Johnston S.S., Chu B.C., Dalal M.R., Schulman K.L. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 3.Khavjou OP D., Leib A. RTI International; Research Triangle Park, NC: November 2016. Projections of Cardiovascular Disease Prevalence and Cost: 2015–2035. [Google Scholar]
- 4.Wilber D.J., Pappone C., Neuzil P., et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 5.Elsayed M., Abdelfattah O.M., Sayed A., et al. Bayesian network meta-analysis comparing cryoablation, radiofrequency ablation, and antiarrhythmic drugs as initial therapies for atrial fibrillation. J Cardiovasc Electrophysiol. 2022;33:197–208. doi: 10.1111/jce.15308. [DOI] [PubMed] [Google Scholar]
- 6.Wynn G.J., Das M., Bonnett L.J., Panikker S., Wong T., Gupta D. Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta-analysis of evidence from randomized and nonrandomized controlled trials. Circ Arrhythm Electrophysiol. 2014;7:841–852. doi: 10.1161/CIRCEP.114.001759. [DOI] [PubMed] [Google Scholar]
- 7.Mont L., Bisbal F., Hernandez-Madrid A., et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study) Eur Heart J. 2014;35:501–507. doi: 10.1093/eurheartj/eht457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C., Zhou X., Zhu M., et al. Catheter ablation versus medical therapy for patients with persistent atrial fibrillation: a systematic review and meta-analysis of evidence from randomized controlled trials. J Interv Card Electrophysiol. 2018;52:9–18. doi: 10.1007/s10840-018-0349-8. [DOI] [PubMed] [Google Scholar]
- 9.Maurer T., Rottner L., Makimoto H., et al. The best of two worlds? Pulmonary vein isolation using a novel radiofrequency ablation catheter incorporating contact force sensing technology and 56-hole porous tip irrigation. Clin Res Cardiol. 2018;107:1003–1012. doi: 10.1007/s00392-018-1270-y. [DOI] [PubMed] [Google Scholar]
- 10.Kautzner J., Neuzil P., Lambert H., et al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace. 2015;17:1229–1235. doi: 10.1093/europace/euv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu M., Han Y., Zhao W., Chen W. Long-term cost-effectiveness comparison of catheter ablation and antiarrhythmic drugs in atrial fibrillation treatment using discrete event simulation. Value Health. 2022;25:975–983. doi: 10.1016/j.jval.2021.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Friedman D.J., Field M.E., Rahman M., et al. Catheter ablation and healthcare utilization and cost among patients with paroxysmal versus persistent atrial fibrillation. Heart Rhythm O2. 2021;2:28–36. doi: 10.1016/j.hroo.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 14.Friberg L., Rosenqvist M., Lip G.Y. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 15.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Burger C.D., Ozbay A.B., Lazarus H.M., et al. Treatment patterns and associated health care costs before and after treatment initiation among pulmonary arterial hypertension patients in the united states. J Manag Care Spec Pharm. 2018;24:834–842. doi: 10.18553/jmcp.2018.17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calkins H., Hindricks G., Cappato R., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladapo J.A., Turakhia M.P., Ryan M.P., Mollenkopf S.A., Reynolds M.R. Health care utilization and expenditures associated with remote monitoring in patients with implantable cardiac devices. Am J Cardiol. 2016;117:1455–1462. doi: 10.1016/j.amjcard.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Field M.E., Gold M.R., Rahman M., et al. Healthcare utilization and cost in patients with atrial fibrillation and heart failure undergoing catheter ablation. J Cardiovasc Electrophysiol. 2020;31:3166–3175. doi: 10.1111/jce.14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarman J.W.E., Hussain W., Wong T., et al. Resource use and clinical outcomes in patients with atrial fibrillation with ablation versus antiarrhythmic drug treatment. BMC Cardiovasc Disord. 2018;18:211. doi: 10.1186/s12872-018-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afzal M.R., Chatta J., Samanta A., et al. Use of contact force sensing technology during radiofrequency ablation reduces recurrence of atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2015;12:1990. doi: 10.1016/j.hrthm.2015.06.026. –1996. [DOI] [PubMed] [Google Scholar]
- 22.Mansour M., Calkins H., Osorio J., et al. Persistent atrial fibrillation ablation with contact force-sensing catheter: the prospective multicenter PRECEPT trial. JACC Clin Electrophysiol. 2020;6:958–969. doi: 10.1016/j.jacep.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Benjamin E.J., Muntner P., Alonso A., et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.