Abstract
Background
Limited real-world data exist on early outcomes in patients with heart failure with preserved ejection fraction (HFpEF) undergoing atrial fibrillation (AF) ablation.
Objectives
The purpose of this study was to examine and compare rates of index procedural complications and 30-day readmissions after AF ablation in patients with HFpEF, with heart failure with reduced ejection fraction (HFrEF), and without heart failure.
Methods
Using the Nationwide Readmissions Database (NRD), we examined 50,299 admissions of adults with heart failure undergoing AF catheter ablation between 2010 and 2014. Using ICD-9-CM codes, we identified procedural complications and causes of readmission after AF ablation.
Results
From 2010 to 2014, the prevalence of HFpEF among patients undergoing AF ablation increased from 3.05% to 7.35% (P for trend <.001). Compared to patients without heart failure, patients with HFpEF had significantly increased procedural complications and index mortality (8.4% vs 6.2% and 0.30% vs 0.08%, respectively; P = .016 and P = .010, respectively). Index complication rates between patients with HFpEF and HFrEF were similar. All-cause 30-day readmissions occurred in 18.3% of patients with HFpEF compared to 9.5% of patients without heart failure (P <.001). Compared to no heart failure, the presence of HFpEF was independently associated with all-cause readmissions (adjusted odds ratio 1.52; 95% confidence interval 1.15–1.96; P = .002), but not with procedural complications, cardiac readmissions, or early mortality.
Conclusion
Rates of 30-day readmissions after AF ablation are high in patients with HFpEF. However, after adjustment for age and comorbidities, complications and early mortality after AF ablation between patients with HFpEF and those without heart failure are comparable.
Keywords: Atrial fibrillation, Catheter ablation, Heart failure with preserved ejection fraction, Heart failure with reduced ejection fraction, Outcomes, Readmissions
Key Findings.
-
▪
The prevalence of heart failure with preserved ejection fraction (HFpEF) among patients undergoing atrial fibrillation (AF) ablation in the real world increased significantly between 2010 and 2014.
-
▪
Patients with HFpEF had more procedural complications, 30-day readmissions, and early mortality after AF ablation compared to patients without heart failure.
-
▪
However, after adjustment for age, sex, comorbidities, and hospital factors, the presence of HFpEF was not independently associated with adverse outcomes after AF ablation.
Introduction
Heart failure is a leading cause of hospitalizations and readmissions in the United States,1 and rates of 30- and 90-day heart failure readmissions have increased from 2010 to 2017.2 Heart failure has been classified as heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF). Importantly, HFpEF is now the most common form of heart failure. Patients with HFpEF tend to be older, be female, and have more comorbidities, including hypertension, renal disease, and atrial fibrillation (AF).3,4 The combination of heart failure and AF is associated with a worse prognosis.5 Catheter ablation has been shown to reduce all-cause mortality, heart failure hospitalizations, and improve left ventricular ejection fraction in those with HFrEF; however, outcomes in HFpEF patients have been less well studied.6, 7, 8 Recent data suggest adoption of an early rhythm control strategy in patients with pre-existing cardiovascular disease, including heart failure, leads to lower risk of adverse cardiovascular outcomes.9 Compared with antiarrhythmic therapy, catheter ablation has demonstrated improvement in quality of life and survival in both HFpEF as well as HFrEF patients.10
Although heart failure has been associated with procedural complications, readmissions, and early mortality after AF ablation,11 real-world short-term outcomes for the subgroup of patients with HFpEF have not been well described. Both single-center studies and meta-analyses have demonstrated that patients with HFpEF have similar freedom from atrial arrhythmia recurrence and symptomatic improvement as HFrEF patients 12 months after catheter ablation.12,13 Using a nationally representative administrative database, we sought to identify complications, early mortality, and 30-day readmissions after AF ablation in patients with HFpEF and to compare these outcomes with those of patients with no heart failure and with HFrEF.
Methods
Data source
All data were obtained from the Agency for Healthcare Research and Quality (AHRQ). The National Readmission Database (NRD) is drawn from the Healthcare Cost and Utilization Project (HCUP) State Inpatient Databases. The NRD is an annual, multipayer administrative database that tracks 1 calendar year of discharge data for patients across hospitals within a state.14, 15, 16 The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for diagnoses and procedures are available for each patient admission record. We used the NRD using the time period from 2010 to 2014. This study duration was prior to 2015 when the “two midnight” rule for the definition of inpatient admissions was enforced. Because the NRD includes only inpatients, NRD studies of AF catheter ablation after 2014 would exclude outpatient procedures and therefore would significantly undersample the number of AF ablations performed. The Weill Cornell Institutional Review Board deemed ethical approval, and informed consent was not required for the study because all data were derived from a de-identified administrative database. The data that support the findings of this study are available from the corresponding author upon reasonable request. The research reported in this paper adhered to the revised 2013 Helsinki Declaration guidelines.
Study population
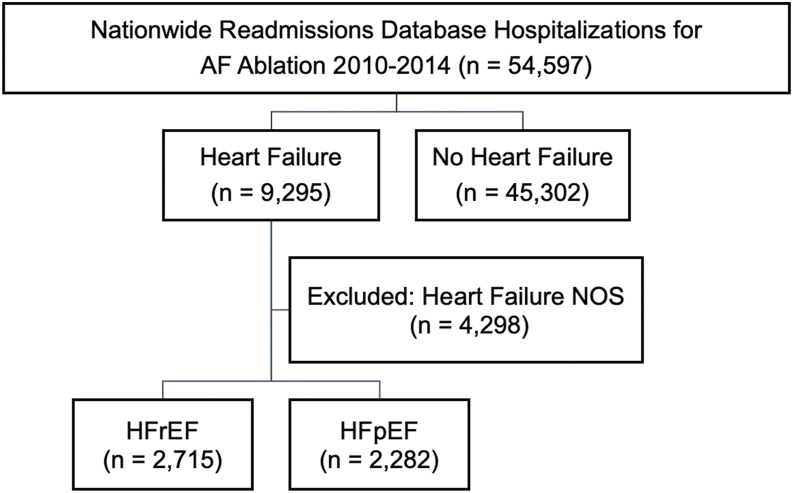
Hospitalizations for catheter ablation of AF were selected by identifying patients with a primary ICD-9-CM diagnosis code for AF (427.31) and a primary ICD-9-CM procedure code for catheter ablation (37.34). Patients with a secondary ICD-9-CM code for other arrhythmias or a procedure code for device implantation during the index admission were excluded (Supplemental Table 1). Heart failure subtype was identified via primary ICD-9-CM code for diastolic heart failure (428.30–428.33) and systolic heart failure (428.20–428.23) and were defined in this study as HFpEF and HFrEF, respectively (Figure 1). Patients with combined systolic and diastolic heart failure (428.40–428.43), unspecified heart failure (428.0, 428.9), and left heart failure (428.1) were excluded (n = 4298) (Supplemental Table 2). Because the NRD is reset annually, we excluded patients if they were discharged in December from their index admission in order to ensure that 30-day follow up after discharge was available. We also excluded patients under the age of 18 years and those without mortality data.
Figure 1.
Flowchart of study population selection. AF = atrial fibrillation; HFpEF = heart failure with preserved ejection fraction; NOS = not otherwise specified; HFrEF = heart failure with reduced ejection fraction.
Clinical variables
We included patient- and hospital-level variables extracted from the NRD, including age, sex, median household income, primary payer, hospital region, and hospital size, for baseline characteristic analysis. Patient-level variables and cardiac diagnoses were defined by ICD-9-CM codes, Clinical Classification Software (CCS) codes, and AHRQ comorbidity measures based on the Elixhauser methods as defined in Supplemental Table 3.
Study endpoints
Our primary endpoints were rates of index procedural complications and 30-day readmissions after AF ablation in patients with HFpEF, HFrEF, and no heart failure, according to the methodology described by HCUP. Procedural complications included cardiac perforation/tamponade, vascular complications, stroke, and other iatrogenic cardiac complications (Supplemental Table 3). ICD-9-CM diagnosis codes were used to identify the causes of 30-day readmissions by organ system (Supplemental Table 4), which included both cardiac and noncardiac causes. Subgroups of cardiac causes of readmissions included atrial fibrillation/tachycardia and congestive heart failure (Supplemental Table 5). In our analysis, we included only the first readmission within 30 days after discharge from the index admission for AF ablation. Index and early mortality included mortality occurring at the index admission or at 30-day readmission, respectively, after AF ablation.
Statistical analysis
All analyses were performed using SAS software, Version 9.4 (SAS Institute, Cary, NC). Discharge weight provided by the NRD was used for all analyses to obtain national estimates. All analyses accounted for hospital-level clustering of patients and complex survey sampling design. Both patient- and hospital-level variables were used for baseline characteristic analysis. For descriptive analyses, we compared baseline patient- and hospital-level variables of AF ablation patients stratified by heart failure phenotype. Categorical variables are given as frequencies, and continuous variables are given as mean (standard error) or median [interquartile range]. Baseline characteristics and outcomes were compared by Rao-Scott χ2 test for categorical variables. Survey-specific linear regression or Mann-Whitney–Wilcoxon nonparametric test was used for continuous variables. To identify predictors of complications, readmissions, and mortality, we created multivariable logistic regression models for the outcome of interest by including covariates that had univariate significance for the outcome (P <.10) (Supplemental Table 6). All P values were corrected for multiple comparisons using the Bonferroni method. All tests were 2-sided, with P <.05 considered significant.
Results
Study population and trends in prevalence of HFpEF among patients undergoing AF ablation
A total of 50,299 admission records from the NRD of patients undergoing catheter ablation of AF from 2010 to 2014 were included in the study analysis. Median length of hospital stay was 1 day [0.4–1.9], suggesting that the majority of AF catheter ablation procedures were overnight stays (Table 1). In this group, 2282 (4.5%) had a diagnosis of HFpEF, 2715 (5.4%) had a diagnosis of HFrEF, and 45,302 (90.0%) had no heart failure (Figure 1). There was a significant increase in the prevalence of heart failure among those undergoing AF catheter ablation during the study period. From 2010 to 2014, the prevalence of HFrEF increased from 3.82% to 7.63% (P for trend <.0001), while the prevalence of HFpEF increased from 3.05% to 7.35% (P for trend <.0001) (Supplemental Figure 1). Compared to patients without heart failure, patients with HFpEF were older, were more likely to be female, and were more likely to have coronary artery disease, previous percutaneous coronary intervention, previous pacemaker implantation, diabetes, obesity, previous stroke, valvular disease, peripheral vascular disease, pulmonary hypertension, and lung disease (Table 1). Patients with HFpEF were less likely to have kidney disease. Compared to patients with HFrEF, patients with HFpEF were older, more likely to be female, and more likely to have previous pacemaker implantation, hypertension, obesity, previous stroke, pulmonary hypertension, and lung disease.
Table 1.
Baseline characteristics of hospitalizations stratified by heart failure subtype
| Characteristics | No CHF | HFrEF | HFpEF |
|---|---|---|---|
| No. of AF ablations | 45,302 | 2715 | 2282 |
| Age (y) [mean (SE)] | 63.4 (0.15) | 68.2 (0.39) | 72.3 (0.39) |
| Age group (y) | |||
| <65 | 22,541 (49.8) | 927 (34.1) | 504 (22.1) |
| 65–74 | 16,263 (35.9) | 910 (33.5) | 777 (34.0) |
| ≥75 | 6498 (14.3) | 878 (32.4) | 1002 (43.9) |
| Female | 16,663 (36.8) | 870 (32.0) | 1403 (61.5) |
| CAD | 10,257 (22.6) | 1411 (52.0) | 927 (40.6) |
| Previous PCI | 3250 (7.1) | 395 (14.6) | 252 (11.0) |
| Previous PPM | 4495 (9.9) | 457 (16.8) | 726 (31.8) |
| Previous ICD | 1092 (2.4) | 957 (35.2) | 95 (4.2) |
| Hypertension | 26,821 (59.2) | 1242 (45.8) | 1279 (56.03) |
| Diabetes mellitus | 8178 (18.1) | 891 (32.8) | 727 (31.9) |
| Hyperlipidemia | 7505 (46.1) | 606 (49.5) | 629 (56.9) |
| Obesity | 6727 (14.8) | 478 (17.6) | 564 (24.7) |
| History of stroke | 2515 (5.6) | 205 (7.6) | 220 (9.7) |
| Valvular disease | 4997 (11.0) | 664 (24.4) | 594 (26.0) |
| Peripheral vascular disease | 1257 (2.8) | 205 (7.6) | 128 (5.6) |
| Pulmonary hypertension | 781 (1.7) | 231 (8.5) | 281 (12.3) |
| Chronic lung disease | 5593 (12.3) | 690 (25.4) | 712 (31.2) |
| Smoking | 2154 (4.8) | 190 (7.0) | 148 (6.5) |
| Renal disease | 1945 (4.3) | 748 (27.5) | 452 (19.8) |
| Alcohol abuse | 477 (1.1) | 56 (2.1) | 27 (1.2) |
| Substance abuse | 164 (0.4) | 24 (0.9) | 8 (0.3) |
| Cancer | 388 (0.9) | 47 (1.7) | 63 (2.8) |
| Anemia | 1983 (4.4) | 320 (11.8) | 359 (15.7) |
| Coagulopathy | 677 (1.5) | 113 (4.2) | 91 (4.0) |
| Elixhauser co-morbidity score >4 | 3248 (7.2) | 1261 (46.4) | 1160 (50.8) |
| Hospital AF ablation volume | |||
| First tertile (lowest) | 15,096 (33.3) | 1387 (51.1) | 1110 (48.6) |
| Second tertile | 15,363 (33.9) | 872 (32.1) | 723 (31.7) |
| Third tertile (highest) | 14,843 (32.8) | 455 (16.8) | 449 (19.7) |
| Teaching hospital | 33,733 (74.5) | 2024 (74.6) | 1627 (71.3) |
| Median household income | |||
| First quartile (lowest) | 8173 (18.3) | 697 (26.0) | 531 (23.6) |
| Second quartile | 10,274 (23.0) | 718 (26.8) | 595 (26.5) |
| Third quartile | 12,098 (27.2) | 614 (22.9) | 510 (22.7) |
| Fourth quartile (highest) | 13,997 (31.4) | 653 (24.3) | 610 (27.2) |
| Primary payer | |||
| Medicare | 22,431 (49.5) | 1929 (71.1) | 1768 (77.5) |
| Medicaid | 1255 (2.8) | 92 (3.4) | 47.7 (2.1) |
| Private including HMO | 20,183 (44.6) | 592 (21.8) | 406 (17.8) |
| Self-pay/no charge/other | 1417 (3.1) | 98 (3.6) | 60 (2.6) |
| Hospital region | |||
| Urban | 44,945 (99.2) | 2678 (98.6) | 2199 (96.4) |
| Hospital bed size | |||
| Small | 1494 (3.3) | 94 (3.5) | 114 (5.0) |
| Medium | 7773 (17.2) | 446 (16.4) | 458 (20.0) |
| Large | 36,035 (79.5) | 2175 (80.1) | 1710 (74.9) |
| Length of stay ≥3 days | 10,670 (23.6) | 1664 (61.3) | 1421 (62.3) |
| LOS, days | 0.89 [0.40–1.92] | 2.83 [1.12–5.63] | 2.88 [1.13–5.84] |
| Cost ($) | 23,199 [16,940–29,773] | 18,685 [10,014–29,284] | 22,728 [13,179–31,355] |
Values are given as n (%) or mean [interquartile range] unless otherwise indicated.
AF = atrial fibrillation; CAD = coronary artery disease; CHF = congestive heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HMO = health maintenance organization; ICD = implantable cardioverter-defibrillator; LOS = length of stay; PCI = percutaneous coronary intervention; PPM = permanent pacemaker.
Rates of AF ablation procedural complications among patients with HFpEF
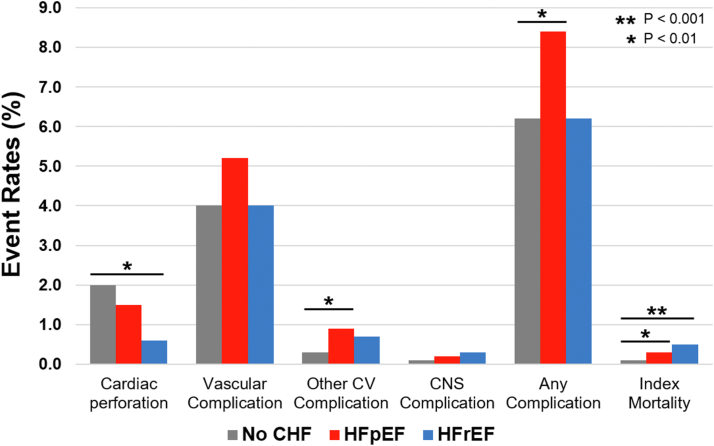
Procedural complications associated with AF ablation, stratified by heart failure type, are shown in Figure 2. Compared to patients without heart failure, patients with HFpEF had higher rates of any procedural complication (8.4% vs 6.2%; P = .016) and index mortality (0.3% vs 0.08%; P = .010). Rates of cardiac perforation (1.5% vs 2.0%; P = .403), vascular complications (5.2% vs 4.0%; P = .099), and central nervous system complications (0.2% vs 0.3%; P = 0.504) were similar between patients with HFpEF and those without heart failure. Compared to patients with HFrEF, patients with HFpEF had a trend toward higher rates of procedural complications (8.4% vs 6.2%; P = .09) but similar rates of index mortality after AF ablation (0.3% vs 0.49%; P = .44).
Figure 2.
Index procedural complications after atrial fibrillation ablation among patients with no heart failure, with heart failure with preserved ejection fraction (HFpEF), and with heart failure with reduced ejection fraction (HFrEF). CHF = congestive heart failure; CNS = central nervous system; CV = cardiovascular.
Thirty-day readmission rates among patients with HFpEF after AF ablation
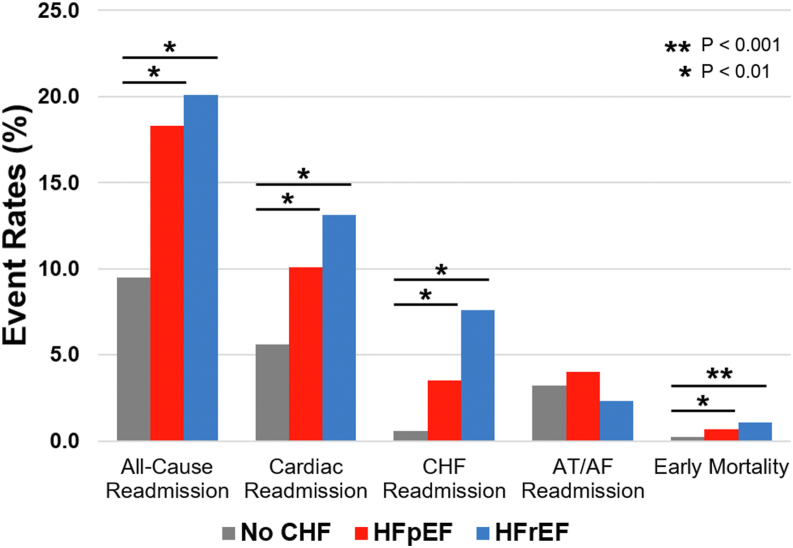
Compared to patients without heart failure, patients with HFpEF had higher rates of all-cause 30-day readmissions (18.3% vs 9.5%; P <.001), cardiac readmissions (10.1% vs 5.6%; P <.001), heart failure readmission (3.5% vs 0.61%; P <.001), noncardiac readmissions (8.2% vs 3.8%; P <.001), and early mortality (0.67% vs 0.25%; P = .007) (Figure 3). Compared to patients with HFrEF, patients with HFpEF had similar rates of 30-day, all-cause readmissions, cardiac readmissions, noncardiac readmissions, and early mortality but fewer heart failure readmissions (3.5% vs 7.6%; P <.001). Readmissions for atrial arrhythmias were similar among patients with HFpEF, with HFrEF, and without heart failure.
Figure 3.
Thirty-day readmissions after atrial fibrillation (AF) ablation among patients with no heart failure, with HFpEF, and with HFrEF. AT = atrial tachycardia. Other abbreviations as in Figure 2.
HFpEF and HPrEF as predictors of outcomes
We used logistic regression analysis to identify the relationship between HFpEF, HFrEF, and index procedural complications as well as 30-day readmissions. After adjusting for age, medical comorbidities, and hospital factors, HFpEF was not independently associated with procedural complications (Table 2) or index mortality (Table 3). With respect to 30-day readmissions, compared to patients without heart failure, HFpEF was independently associated with all-cause readmissions (adjusted odds ratio [aOR] 1.52; 95% confidence interval [CI]1.17–1.99; P <.01) and heart failure readmissions (aOR 2.42; 95% CI 1.51–3.86; P <.001) but not cardiac readmission, noncardiac readmissions, or early mortality.
Table 2.
Association between heart failure subtype and index procedural complications (absence of CHF as reference)
| Outcome | Unadjusted OR (95% CI) | Unadjusted P value | Adjusted OR (95% CI) | Adjusted P value |
|---|---|---|---|---|
| Cardiac perforation | ||||
| No HF | Reference | Reference | ||
| HFrEF | 0.28 (0.14–0.57) | <.001 | 0.31 (0.15–0.65) | .002 |
| HFpEF | 0.75 (0.39–1.46) | .404 | 0.68 (0.35–1.29) | .236 |
| Vascular complication | ||||
| No HF | Reference | Reference | ||
| HFrEF | 1.00 (0.72–1.39) | .988 | 0.89 (0.59–1.35) | .579 |
| HFpEF | 1.30 (0.95–1.78) | .099 | 0.82 (0.58–1.17) | .269 |
| Other CV complication | ||||
| No HF | Reference | Reference | ||
| HFrEF | 1.88 (1.21–2.92) | .005 | 1.55 (0.99–2.45) | .058 |
| HFpEF | 2.13 (1.03–4.41) | .041 | 1.77 (0.85–3.68) | .128 |
| CNS complication | ||||
| No HF | Reference | Reference | ||
| HFrEF | 0.99 (0.34–2.82) | .978 | 1.01 (0.35–2.90) | .992 |
| HFpEF | 0.61 (0.14–2.61) | .508 | 0.59 (0.14–2.53) | .479 |
| Any complication | ||||
| No HF | Reference | Reference | ||
| HFrEF | 1.00 (0.77–1.31) | .986 | 0.91 (0.67–1.24) | .554 |
| HFpEF | 1.39 (1.06–1.81) | .017 | 0.95 (0.71–1.28) | .758 |
| Index mortality | ||||
| No HF | Reference | Reference | ||
| HFrEF | 6.09 (2.33–15.89) | <.001 | 1.98 (0.60–6.53) | .264 |
| HFpEF | 3.76 (1.27–11.14) | .0168 | 1.12 (0.40–3.55) | .758 |
CI = confidence interval; CNS = central nervous system; CV = cardiovascular; HF = heart failure; OR = odds ratio; other abbreviations as in Table 1.
Table 3.
Association between heart failure subtype and 30-day readmissions (absence of CHF as reference)
| Outcome | Unadjusted OR (95% CI) | Unadjusted P value | Adjusted OR (95% CI) | Adjusted P value |
|---|---|---|---|---|
| All-cause readmission | ||||
| No HF | Reference | Reference | ||
| HFrEF | 2.39 (1.97–2.90) | <.001 | 1.56 (1.19–2.05) | .001 |
| HFpEF | 2.14 (1.79–2.55) | <.001 | 1.52 (1.17–1.98) | .002 |
| Cardiac readmission | ||||
| No HF | Reference | Reference | ||
| HFrEF | 2.52 (2.03–3.12) | <.001 | 1.98 (1.44–2.73) | <.001 |
| HFpEF | 1.88 (1.52–2.34) | <.001 | 1.36 (0.98–1.93) | .064 |
| HF readmission | ||||
| No HF | Reference | Reference | ||
| HFrEF | 13.25 (9.52–18.46) | <.001 | 5.99 (4.21–8.53) | <.001 |
| HFpEF | 6.04 (4.10–8.88) | <.001 | 2.42 (1.51–3.86) | <.001 |
| AT/AF readmission | ||||
| No HF | Reference | Reference | ||
| HFrEF | 0.71 (0.49–1.05) | .085 | 1.07 (0.59–1.94) | .882 |
| HFpEF | 1.24 (0.86–1.77) | .246 | 0.93 (0.55–1.56) | .772 |
| Noncardiac readmission | ||||
| No HF | Reference | Reference | ||
| HFrEF | 1.86 (1.39–2.48) | <.001 | 1.12 (0.80–1.58) | .520 |
| HFpEF | 2.23 (1.70–2.90) | <.001 | 1.25 (0.94–1.67) | .124 |
| Early mortality | ||||
| No HF | Reference | Reference | ||
| HFrEF | 4.39 (2.34–8.23) | <.001 | 1.79 (0.66–4.88) | .252 |
| HFpEF | 2.66 (1.26–5.60) | <.001 | 0.95 (0.42–2.16) | .904 |
Discussion
Using a nationally representative, all-payer administrative database, we identified several important findings in this real-world analysis of early outcomes after AF catheter ablation in HFpEF patients. First, the prevalence of HFpEF among patients undergoing AF ablation increased significantly between 2010 and 2014. Second, compared to patients without heart failure, patients with HFpEF had more procedural complications, all-cause 30-day readmissions, cardiac readmissions, noncardiac readmissions, and early mortality after AF ablation compared to patients without heart failure. After adjustment for age, sex, comorbidities, and hospital factors, the presence of HFpEF was independently associated with all-cause and heart failure readmissions but not independently associated with procedural complications, cardiac readmissions, or early mortality. Our study suggests that many adverse outcomes seen in patients with HFpEF undergoing AF ablation are driven by age, sex, and comorbidities rather than the presence of HFpEF itself.
In patients with left ventricular systolic dysfunction, AF ablation can improve left ventricular ejection fraction, exercise capacity, and quality of life, as well as reduce hospitalizations and mortality.6, 7, 8 Studies of AF ablation outcomes are more limited in patients with HFpEF. There are some phenotypic differences between patients with AF who have HFpEF vs those with HFrEF. In a study by Melenovsky et al17 that evaluated echocardiographic and catheterization parameters of the left atrium in 198 heart failure patients and 40 heart failure-free controls, both HFrEF and HFpEF patients had more dilated atria and greater degrees of left atrial dysfunction. Compared to patients with HFrEF, patients with HFpEF can have a higher burden of AF due to higher left atrial peak pressures and higher left atrial stiffness. Among patients with HFpEF, left atrial dysfunction is associated with increased mortality. Several smaller studies have shown that catheter ablation of AF in patients with HFpEF can be safe and is associated with reduced heart failure events. In an observational, single-center, retrospective cohort study, Aldaas et al18 examined outcomes of 547 patients, including 51 (9%) with HFpEF and 40 (7%) with HFrEF, who underwent radiofrequency catheter ablation for AF. There were no significant differences in recurrence of atrial arrhythmias, procedural complications, or 5-year survival in patients with HFpEF vs patients with HFrEF vs patients without heart failure. All-cause hospitalizations were more common in HFpEF and HFrEF patients than in those without heart failure.18
Larger-scale, prospective, randomized clinical trials of outcomes after AF catheter ablation in patients with HFpEF are needed. Single-center studies to date, such as STALL AF-HFpEF (A Prospective Study Using Invasive Haemodynamic Measurements Following Catheter Ablation for AF and Early HFpEF), which examined 54 patients with AF, 35 of whom also had HFpEF, have demonstrated that AF catheter ablation can lead to improvements in quality of life and hemodynamic parameters such as postexercise pulmonary capillary wedge pressure in patients with HFpEF.19 Additional prospective trials seek to examine various outcomes in HFpEF patients undergoing AF catheter ablation, including exercise capacity and quality of life20 and arrhythmia recurrence,21 are currently under recruitment. Once these data are available, there may be further expansion in the patient population offered early AF ablation.
Our study provides real-world insight into short-term outcomes after AF catheter ablation in patients with HFpEF, and its findings suggest that HFpEF does not seem to independently confer increased rates of procedural complications, index procedural mortality, or early mortality. However, as with HFrEF, HFpEF is independently associated with increased risk of all-cause 30-day readmissions. Although there was a trend toward HFpEF as an independent predictor of cardiac readmissions, this was not seen with regard to readmissions for atrial tachycardia/AF recurrence. Taken together, these findings underscore the importance of patient optimization by electrophysiologists and heart failure specialists in the periprocedural period.
Study limitations
This was a retrospective study based on administrative data from the NRD, which relies on entered ICD-9-CM codes. This introduces potential error from miscoding, undercoding or overcoding, and missing data. Notably, the ICD-9-CM does not include specific codes for HFrEF or HFpEF, so codes for systolic and diastolic heart failure, respectively, were used as surrogate identifiers of these conditions. In recent years, efforts have been made to standardize the definition of HFpEF using scoring systems. Reddy et al22 created the H2FPEF score, which utilizes clinical and echocardiographic criteria to discern HFpEF from noncardiac causes of dyspnea. In addition, the European Society of Cardiology created the HFA-PEFF score, which uses biomarker as well as functional and morphologic echocardiographic criteria, in an algorithm used to diagnose HFpEF.23 Because echocardiographic parameters were not available in the NRD, these scoring systems could not be used to further ascertain the diagnosis of HFpEF in our study population. Given the likely underestimation of HFpEF prevalence in our study, complication and readmission rates may be overestimated. With publication of studies showing improved outcomes with AF ablation for patients with HFrEF,6 a more contemporary analysis of patients with heart failure undergoing AF ablation may reveal different patient characteristics. The NRD does not include data on clinical variables such as New York Heart Association (NYHA) functional class, atrial fibrillation burden, and medications; therefore, we were unable to explore the impact of these patient factors on outcomes. Procedural variables such as type of catheter ablation, procedural length, and operator expertise, also are not included in the NRD. There have been numerous advances in the practice of AF catheter ablation since this study period. Improvements in catheter-based technologies have led to greater procedural safety, shorter procedural durations, and more durable pulmonary vein isolation.24 In addition, emerging technologies such as irreversible electroporation have shown promise in early trials, with larger-scale data on safety and efficacy forthcoming.25 Given this, the readmissions and procedural complications rates seen in our study are expected to be higher than those seen with a contemporary cohort. Although the NRD is designed to approximate the national distribution of hospital characteristics, it does not include data from all 50 states in the United States; therefore, certain hospital types may be overrepresented or underrepresented, thus limiting the generalizability of this study. Moreover, given that the NRD is an inpatient database, variability in coding of inpatient vs outpatient procedures can lead to heterogeneity in patient inclusion across institutions. Specifically, the exclusion of patients undergoing AF catheter ablation as outpatients may have biased our study population. Finally, estimates of early mortality may be underrepresented because out-of-hospital deaths before readmission would not be included in the analysis.
Conclusion
In this nationally representative real-world cohort, rates of 30-day readmissions after AF catheter ablation are higher in patients with HFpEF than in patients without heart failure. After adjustment, HFpEF was not an independent predictor of overall index procedural complications, cardiac readmissions, index mortality, or early mortality after AF catheter ablation. Further studies examining the role of patient and procedural factors are warranted to determine the clinical predictors of readmissions in heart failure patients after AF catheter ablation. A multidisciplinary approach to optimize periprocedural heart failure management may reduce readmissions in patients with HFpEF who undergo catheter ablation of AF.
Acknowledgments
Funding Sources
This work was supported by grants from the Michael Wolk Heart Foundation, the New York Cardiac Center, Inc., and the New York Weill Cornell Medical Center Alumni Council. The Michael Wolk Heart Foundation, the New York Cardiac Center, Inc., and the New York Weill Cornell Medical Center Alumni Council had no role in the design and conduct of the study, the collection, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript.
Disclosures
Dr Cheung has received consulting fees from Abbott, Biotronik, Boston Scientific, and Zoll Medical; research grant support from Boston Scientific; and fellowship grant support from Abbott, Biosense Webster, Biotronik, Boston Scientific, and Medtronic. Dr Markowitz has received consulting fees from Preventice Medical and fees from Boston Scientific for serving on a data safety monitoring board. Dr Goyal is supported by American Heart Association Grant 20CDA35310455, National Institute on Aging Grant K76AG064428, and Loan Repayment Program award L30AG060521; receives personal fees for medicolegal consulting related to heart failure; and has received honoraria from Akcea Therapeutics Inc and Bionest Inc. Dr Horn has received fees from V-wave Ltd and SoniVie for serving on a data safety monitoring board and clinical events committee. All other authors report no relevant disclosures.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Informed consent was not required for the study because all data were derived from a de-identified administrative database.
Ethics Statement
The Weill Cornell Institutional Review Board deemed ethical approval, and informed consent was not required for the study because all data were derived from a de-identified administrative database. The research reported in this paper adhered to the revised 2013 Helsinki Declaration guidelines.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2022.06.012.
Supplementary data
References
- 1.Ambrosy A.P., Fonarow G.C., Butler J., et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Khan M.S., Sreenivasan J., Lateef N., et al. Trends in 30- and 90-day readmission rates for heart failure. Circ Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.121.008335. [DOI] [PubMed] [Google Scholar]
- 3.Goyal P., Almarzooq Z.I., Horn E.M., et al. Characteristics of hospitalizations for heart failure with preserved ejection fraction. Am J Med. 2016;129:635. doi: 10.1016/j.amjmed.2016.02.007. e15–e26. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg B.A., Zhao X., Heidenreich P.A., et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 5.Goyal P., Almarzooq Z.I., Cheung J., et al. Atrial fibrillation and heart failure with preserved ejection fraction: Insights on a unique clinical phenotype from a nationally-representative United States cohort. Int J Cardiol. 2018;266:112–118. doi: 10.1016/j.ijcard.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrouche N.F., Kheirkhahan M., Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379:492. doi: 10.1056/NEJMc1806519. [DOI] [PubMed] [Google Scholar]
- 7.Geng J., Zhang Y., Wang Y., et al. Catheter ablation versus rate control in patients with atrial fibrillation and heart failure: a multicenter study. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000009179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prabhu S., Taylor A.J., Costello B.T., et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol. 2017;70:1949–1961. doi: 10.1016/j.jacc.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Kirchhof P., Camm A.J., Goette A., et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 10.Packer D.L., Piccini J.P., Monahan K.H., et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143:1377–1390. doi: 10.1161/CIRCULATIONAHA.120.050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng E.P., Liu C.F., Yeo I., et al. Risk of mortality following catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2019;74:2254–2264. doi: 10.1016/j.jacc.2019.08.1036. [DOI] [PubMed] [Google Scholar]
- 12.Black-Maier E., Ren X., Steinberg B.A., et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm. 2018;15:651–657. doi: 10.1016/j.hrthm.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Aldaas O.M., Lupercio F., Darden D., et al. Meta-analysis of the usefulness of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2021;142:66–73. doi: 10.1016/j.amjcard.2020.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agency for Healthcare Research and Quality Introduction to the HCUP Nationwide Readmissions Database (NRD). 2010–2015. https://www.hcup-us.ahrq.gov/db/nation/nrd/Introduction_NRD_2010-2015.pdf Available from.
- 15.Healthcare Cost and Utilization Project (HCUP) Overview of the Nationwide Readmissions Database (NRD) http://www.hcup-us.ahrq.gov/nrdoverview.jsp Available from.
- 16.HCUP Quality Control Procedures Deliverable #1707.05. https://www.hcup-us.ahrq.gov/db/quality.pdf Available from.
- 17.Melenovsky V., Hwang S.J., Redfield M.M., Zakeri R., Lin G., Borlaug B.A. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 18.Aldaas O.M., Malladi C.L., Mylavarapu P.S., et al. Comparison of outcomes after ablation of atrial fibrillation in patients with heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2020;136:62–70. doi: 10.1016/j.amjcard.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugumar H., Nanayakkara S., Vizi D., et al. A prospective STudy using invAsive haemodynamic measurements foLLowing catheter ablation for AF and early HFpEF: STALL AF-HFpEF. Eur J Heart Fail. 2021;23:785–796. doi: 10.1002/ejhf.2122. [DOI] [PubMed] [Google Scholar]
- 20.Ablation Versus Medical Management of Atrial Fibrillation in HFpEF (AMPERE) https://clinicaltrials.gov/ct2/show/NCT04282850 Available from.
- 21.Catheter Ablation for Atrial Fibrillation in Preserved Ejection Fraction (AFFECT) https://clinicaltrials.gov/ct2/show/NCT04317911 Available from.
- 22.Reddy Y.N.V., Carter R.E., Obokata M., Redfield M.M., Borlaug B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieske B., Tschöpe C., de Boer R.A., et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 24.Parameswaran R., Al-Kaisey A.M., Kalman J.M. Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nat Rev Cardiol. 2021;18:210–225. doi: 10.1038/s41569-020-00451-x. [DOI] [PubMed] [Google Scholar]
- 25.Loh P., van Es R., Groen M.H.A., et al. Pulmonary vein isolation with single pulse irreversible electroporation: a first in human study in 10 patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.008192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.