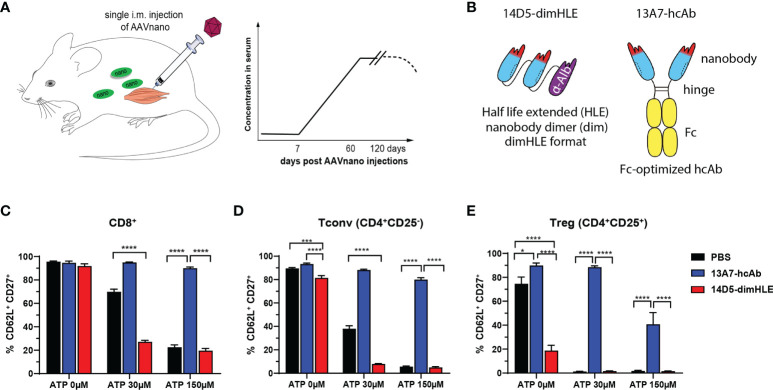
Figure 1.
AAVnano methodology used to block or to potentiate P2X7 activity in vivo (A) AAVnano methodology is based on the single i.m. injection of AAVnano vectors coding for a nanobody-based biologic. This is anticipated to induce long-term and stable in vivo production of the designed biologics in vivo (43). (B) Schemes illustrating the format of the different nanobody-based biologics used in this work. The 14D5-dimHLE is composed of a dimer of the P2X7-potentiating 14D5 nanobody, coupled to a third albumin-specific nanobody (Alb8) conferring extended half-life (HLE). The 13A7-hcAb is composed of an antagonistic nanobody targeting P2X7, coupled to the hinge and the Fc-region of a mouse IgG1, carrying “LSF” mutations (T252L, T254S, T256F) to confer higher affinity to the neonatal Fc receptor (FcRn) involved in extending antibody half-life in vivo (50). (C–E) The ability of the corresponding AAVnano vectors to induce functional modulation (i.e. inhibition or potentiation) of P2X7 functions in vivo, was assessed 20 days after their i.m. injection. For that, blood samples were collected and incubated in vitro with 0, 30 or 150 µM ATP. P2X7-dependent shedding of CD62L and CD27 was evaluated at the surface of CD8+, CD4+CD25- (Tconv), and CD4+CD25+ (Tregs) lymphocyte subsets, known to express different level of P2X7 and to display increasing sensitivity to ATP. One representative experiment out of at least two is shown with n=7 mice per group. The statistical comparisons between groups were performed using one-way ANOVA. *p<0.05, ***p<0.001, ****p<0.0001.