Abstract
Coronary Artery Disease Reporting and Data System (CAD-RADS) was created to standardize reporting system for patients undergoing coronary CT angiography (CCTA) and to guide possible next steps in patient management. The goal of this updated 2022 CAD-RADS 2.0 is to improve the initial reporting system for CCTA by considering new technical developments in Cardiac CT, including data from recent clinical trials and new clinical guidelines. The updated CAD-RADS classification will follow an established framework of stenosis, plaque burden, and modifiers, which will include assessment of lesion-specific ischemia using CT fractional-flow-reserve (CT-FFR) or myocardial CT perfusion (CTP), when performed. Similar to the method used in the original CAD-RADS version, the determinant for stenosis severity classification will be the most severe coronary artery luminal stenosis on a per-patient basis, ranging from CAD-RADS 0 (zero) for absence of any plaque or stenosis to CAD-RADS 5 indicating the presence of at least one totally occluded coronary artery. Given the increasing data supporting the prognostic relevance of coronary plaque burden, this document will provide various methods to estimate and report total plaque burden. The addition of P1 to P4 descriptors are used to denote increasing categories of plaque burden. The main goal of CAD-RADS, which should always be interpreted together with the impression found in the report, remains to facilitate communication of test results with referring physicians along with suggestions for subsequent patient management. In addition, CAD-RADS will continue to provide a framework of standardization that may benefit education, research, peer-review, artificial intelligence development, clinical trial design, population health and quality assurance with the ultimate goal of improving patient care.
Keywords: Coronary Artery Disease, Coronary CTA, CAD-RADS, Reporting and Data System, Stenosis Severity, Report Standardization Terminology, Plaque Burden, Ischemia
Supplemental material is available for this article.
This article is published synchronously in Radiology: Cardiothoracic Imaging, Journal of Cardiovascular Computed Tomography, JACC: Cardiovascular Imaging, Journal of the American College of Radiology, and International Journal for Cardiovascular Imaging.
© 2022 Society of Cardiovascular Computed Tomography. Published by RSNA with permission.
Keywords: Coronary Artery Disease, Coronary CTA, CAD-RADS, Reporting and Data System, Stenosis Severity, Report Standardization Terminology, Plaque Burden, Ischemia
1. Introduction
Coronary CT angiography (CCTA) has undergone significant technical advancements and clinical validation in the last decade, and several professional societies have issued guidelines, expert consensus documents, and Appropriateness Criteria for CCTA (1–11). Training physicians and technologists in image acquisition and interpretation is essential for fostering quality (12). Such training should also include an approach to standardized reporting in order to decrease variability among practitioners and ensure that test results are appropriately used in patient management decisions.
The purpose of this document is to update the first version of the CAD-RADS (13) standardized classification of coronary artery disease for patients undergoing CCTA that was originally published in 2016 in order to include additional features such as plaque burden and ischemia, and to incorporate evidence from recent clinical trials as well as new clinical practice guidelines. The updated 2022 CAD-RADS 2.0 classification will follow a framework of stenosis, plaque burden and modifiers, with the option to also include ischemia evaluation by CT fractional-flow-reserve (CT-FFR) or myocardial CT perfusion (CTP), if performed. As in the original version, the most severe coronary artery luminal stenosis defined on a per-patient basis will be the central component of assessment and will provide the numeric descriptor. In addition, methods to estimate, quantify and report overall plaque burden will be provided. Collectively, the goal of these additions is intended to enhance patient management decisions following CCTA.
The main goal of CAD-RADS remains to standardize reporting of CCTA results and to facilitate communication of test results to referring physicians along with suggestions for subsequent patient management. Importantly, CAD-RADS should not be viewed as a substitute for the impression section of the report provided by the reading physician. CAD-RADS provides a complementary assessment and should always be interpreted in conjunction with the more detailed and patient-specific information found in the report and the impression, particularly because the report may provide more specific information regarding the location and extent of coronary plaque and stenosis. Furthermore, the clinical management suggestions provided by CAD-RADS should not replace clinical judgment, particularly as there are many patient-specific factors that may influence clinical management.
2. Clinical value of CAD-RADS and coronary CT angiography
More than 50 publications have further validated specific aspects of CAD-RADS since its original publication in 2016 (14) (Fig 1). The CAD-RADS classification has been shown to accurately predict major adverse cardiovascular events, defined as unstable angina, myocardial infarction, or death, in patients with stable chest pain with superior performance when compared with traditional risk factors, other risk stratification scores, the Coronary Artery Calcium Score (CAC) and the earlier SCCT coronary stenosis scoring system (15–19). CAD-RADS has also been demonstrated to correlate with the degree of stenosis measured by invasive coronary angiography (ICA) with high diagnostic accuracy (20,21). Recent publications have highlighted that adoption of CAD-RADS in clinical practice results in reduced downstream testing and cardiology referral rates in patients with non-obstructive coronary artery disease (22) and has a favorable impact on medical therapy and systolic blood pressure control (23). Finally, recent studies have validated the performance of deep learning algorithms for the evaluation of CAD-RADS classification on CCTA (24).
Figure 1:
Timeline plots of total quarterly PubMed citations resulting from the search “CAD-RADS” [Title/Abstract] OR “CADRADS” [Title/Abstract]. The date of the search was January 25, 2021. Permission received (63). Radiol Cardiothorac Imaging. 2021 Jun; 3 (3): e210016.
There has been widespread adoption of CAD-RADS in clinical practice with most sites in the United States and around the world using this classification for reporting CCTA on a routine basis. Overall, available research suggests that CAD-RADS offers a clinically useful and appropriate categorization of coronary artery disease with high diagnostic accuracy when compared with invasive angiography, with robust prognostic value and a beneficial impact on medical management.
Since the publication of the original CAD-RADS classification, several prospective trials have provided evidence supporting the clinical utility of CCTA and the relevance of CT findings among patients with suspected stable coronary artery disease. They include the PROMISE (25) and SCOT-HEART (26) trials, which demonstrated that CCTA is clinically useful as an alternative to functional testing (PROMISE) or in addition to standard of care (SCOT-HEART). Based on these trials and multiple registries, the prognostic value of the CAD-RADS classification has been confirmed, demonstrating that higher CAD-RADS scores were associated with increased risks of fatal and non-fatal MI (15–17).
Moreover, several large randomized trials (CT-STAT, ACRIN-PA, ROMICAT II, CT-COMPARE) have compared CCTA to the current standard of care in patients with acute chest pain (27–30). Complemented by real world implementation data (31–33), they consistently demonstrated the safety of discharging patients from the emergency department based on a negative CCTA, resulting in guidelines supporting the use of CCTA in low to intermediate risk patients presenting with acute chest pain to the emergency department (34).
CCTA is now considered a first-line test (Class I) for use in acute and chronic coronary syndromes by the European Society of Cardiology (11), NICE guideline (10) and by the new American College of Cardiology and American Heart Association Chest Pain Guideline (35), particularly in symptomatic patients with stable symptoms and intermediate or high pre-test probability of obstructive coronary artery disease, or among intermediate-risk acute chest pain patients. Moreover, there have been numerous advances in the detection and quantification, understanding of atherosclerotic plaque burden by CCTA, as well as a better understanding of the clinical implications of various CCTA findings (36).
Despite the robust evidence base supporting the use of CCTA in patients with acute and stable chest pain, there is insufficient prospective randomized clinical trial data to support the optimal clinical management strategy following CCTA. Accordingly, the CAD-RADS classification is an expert consensus document. As such, the recommendations provided in this document are based on the available research data from clinical trials as well as on broad expert consensus. This includes the suggested categories for reporting and the recommendations for further patient management, which need to be interpreted in the context of other available clinical information for each individual patient.
3. CAD-RADS reporting system
3.1. CAD-RADS categories
CAD-RADS categories are based on stenosis severity and plaque burden. For the grading of stenosis severity, a classification system originally developed by the Society of Cardiovascular Computed Tomography is used (see Table 1). Table 1 also describes the terminology used to estimate the overall amount of plaque burden (P1 to P4) and the classification of ischemia into positive (I+), negative (I−) or borderline (I+/−), if a CT-based ischemia test such as, FFR-CT or myocardial CTP has been performed. Table 2 describes selected methods to categorize the overall amount of coronary plaque by CCTA. Table 3 describes examples of non-atherosclerotic causes of coronary abnormalities to be included in Modifier “E” = Exceptions. Tables 4 and 5 list the categories of the CAD-RADS reporting system for stable chest pain (Table 4) and acute chest pain (Table 5) with suggestions for further cardiac investigation and management considerations. In both settings, they range from CAD-RADS 0 (absence of atherosclerosis) to CAD-RADS 5 (presence of at least one total vessel occlusion). Figures 2 through 6 provide examples of different amounts of plaque burden and associated categories and terminology. Figures 7 through 12 provide examples of CAD-RADS categories 4A, 4B, 5 and N.
Table 1:
Grading scale for stenosis severity, plaque burden and ischemia.
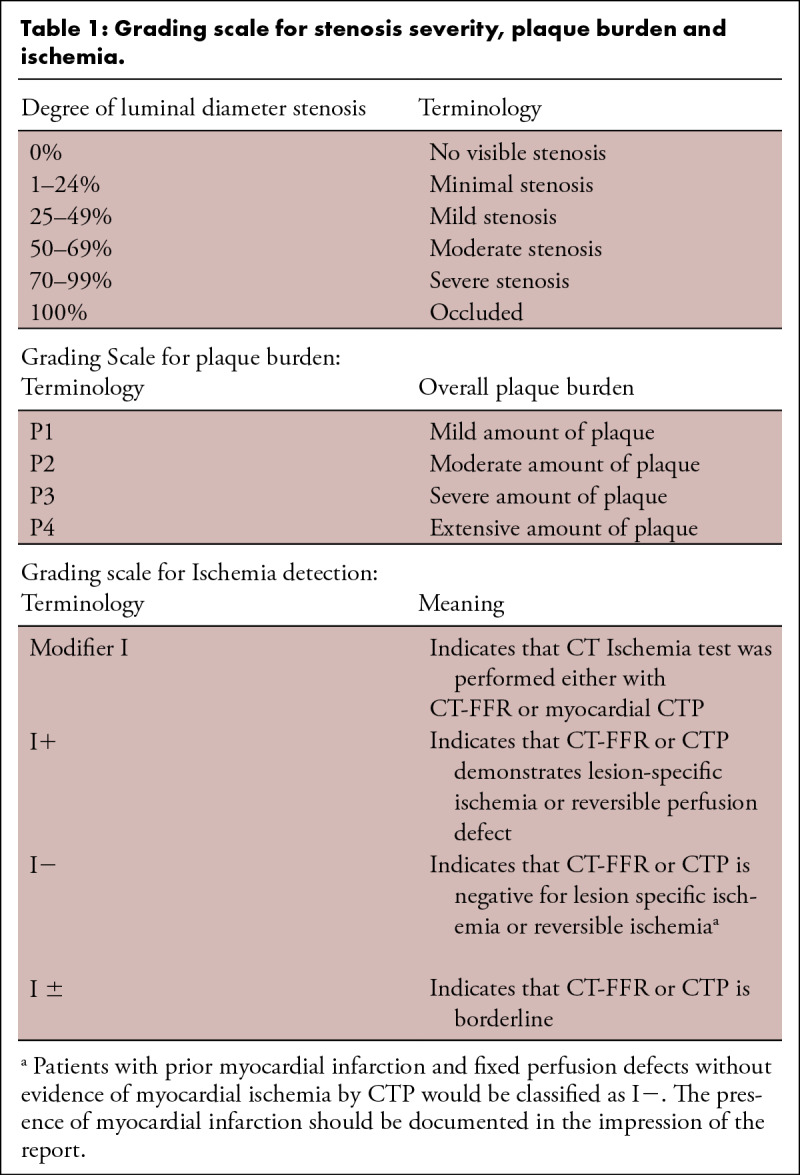
Table 2:
Different methods to categorize the overall amount of coronary plaque.
Table 3:
Examples of non-atherosclerotic causes of coronary abnormalities to be included in Modifier “E” = Exceptions. Please note that this is not a comprehensive list.
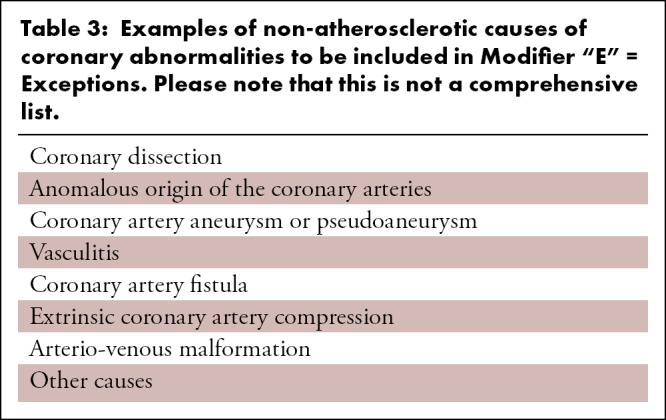
Table 4:
CAD-RADS Reporting and Data System for patients presenting with stable chest pain.
Table 5:
CAD-RADS Reporting and Data System for patients presenting with acute chest pain.
Figure 2:
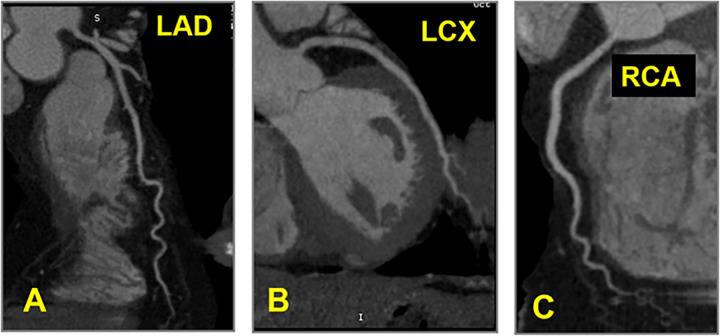
CAD-RADS 0 – No coronary stenosis. Absence of calcified and non-calcified plaque in the coronary tree. The classification P is not required for CAD-RADS 0.
Figure 6:
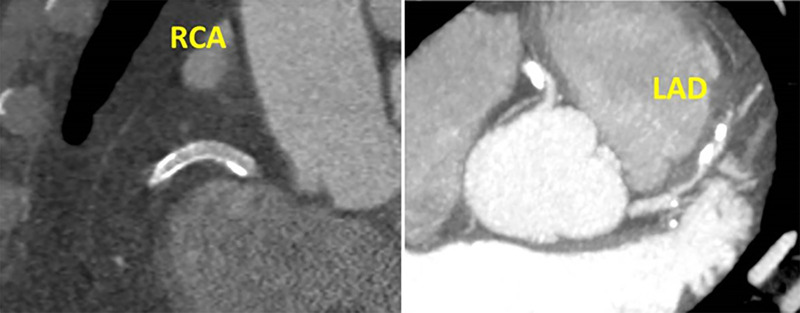
CAD-RADS 4B/P4. Plaque Burden – P4: Three vessel severe coronary stenosis with extensive amount of plaque burden – CAC = 3607.
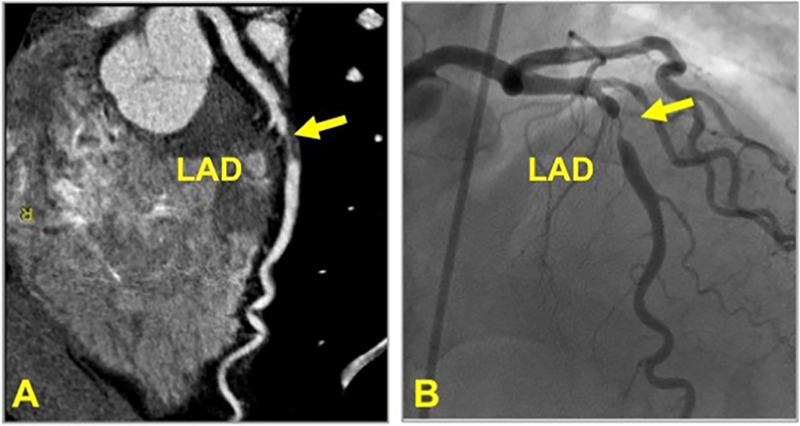
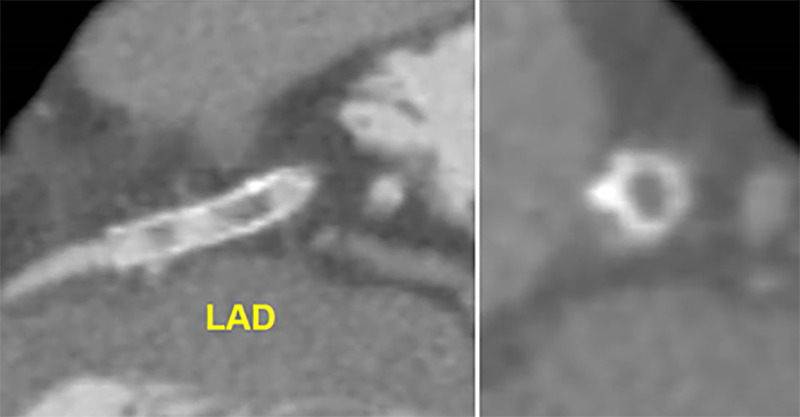
Figure 7:
CAD-RADS 4A/P1. Focal non-calcified plaque in the mid LAD (yellow arrow) with 70–99% severe coronary stenosis and mild amount of focal non-calcified plaque burden (P1) (left). Invasive coronary angiography confirming 70–99% stenosis in the mid LAD (yellow arrow, right). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Figure 12:
CAD-RADS 3/P2/N. Motion artifact obscuring the mid RCA (left, arrow), which renders this segment non-diagnostic. There is also stenosis of the mid LAD with 50–69% luminal narrowing (right, arrow), qualifying this lesion as CAD RADS 3 and moderate amount of coronary plaque (P2). Although the mid RCA segment is non-diagnostic, the presence of suspected obstructive disease within the LAD should be coded as CAD RADS 3/P2/N. If the LAD lesion were mild (less than 50% diameter stenosis), and no other stenosis were identified, the patient would be coded as CAD RADS N.
Figure 3:
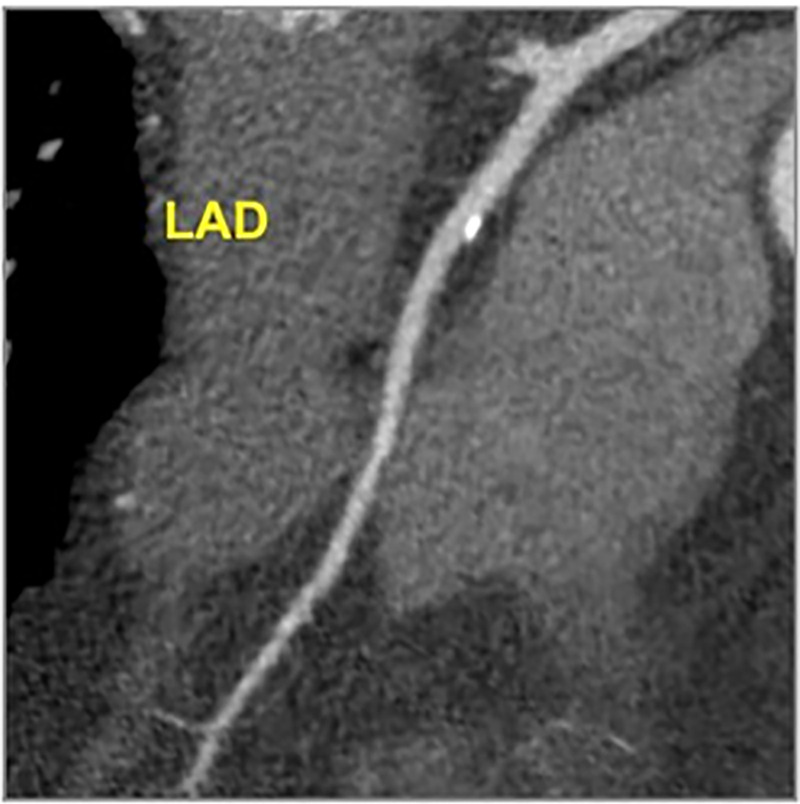
CAD-RADS 1/P1 - Minimal coronary stenosis (1–24%). Plaque Burden –P1: Mild amount of plaque burden.
Figure 4:
CAD-RADS 2/P2 – Mild coronary stenosis (25–49%). Plaque Burden – P2: Moderate amount of plaque burden.
Figure 5:
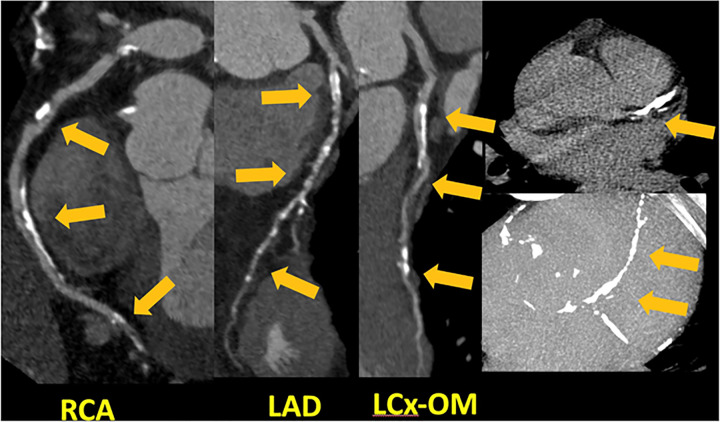
CAD RADS 1/P3 - Plaque Burden – P3: Severe amount of plaque burden – SIS = 7, Extensive amount of diffuse plaque and minimal coronary stenosis.
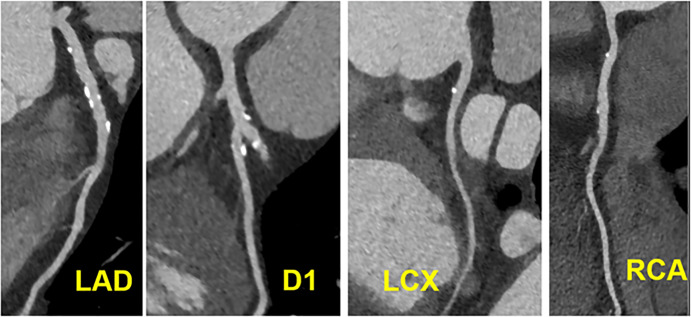
Figure 8:
CAD-RADS 4B/P2. Three-vessel obstructive disease (>70% stenosis), including in 70–99% stenosis of the proximal RCA (left), 70–99% stenosis of the proximal LAD (middle) and 70–99% stenosis of the mid LCX (right) and moderate amount of non-calcified plaque burden (P2).
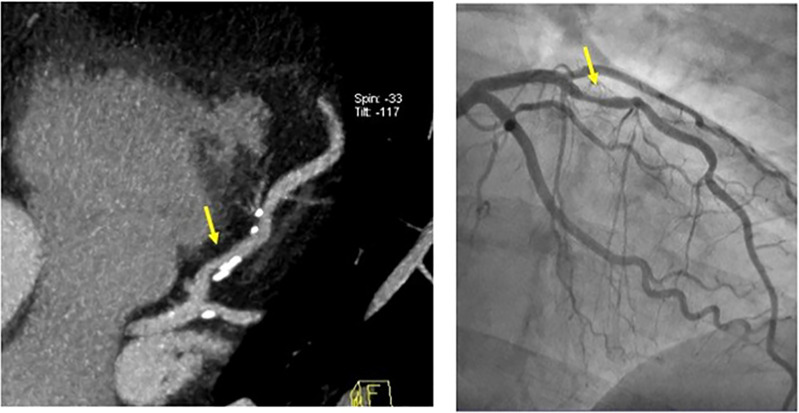
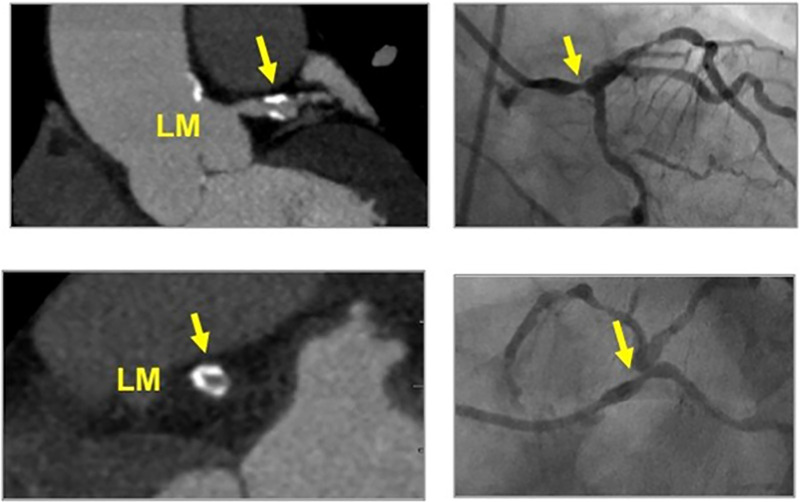
Figure 9:
CAD-RADS 4B/P3. Distal left main stenosis with circumferential calcified plaque resulting in >50% stenosis (arrow) and severe amount of plaque (P3 - Calcium Score = 640). Upper left panel: oblique longitudinal plane of the left main coronary artery. Lower left panel – cross-sectional slice of the distal left main coronary artery. Figures on the right - Invasive coronary angiography confirming focal severe stenosis in the distal left main coronary artery.
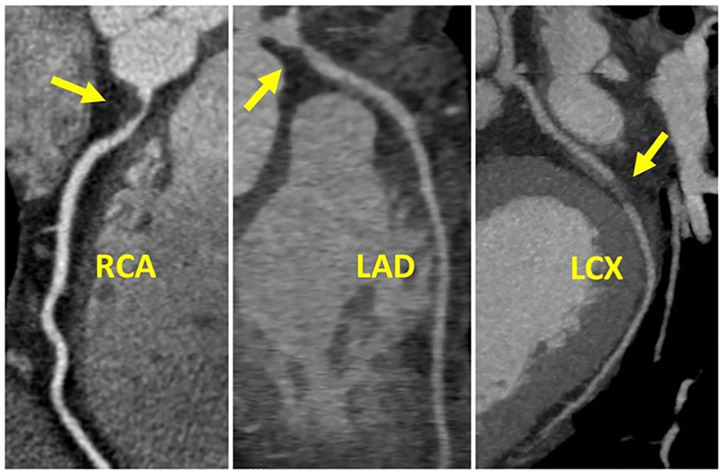
Figure 10:
CAD-RADS 5/P3. Two examples of 5 mm thick MIPs CCTA cases coded as CAD-RADS 5. Left: Focal, non-calcified occlusion of the proximal RCA (arrow) and severe amount of plaque (P3). Right: Total occlusion of the proximal LCX (arrow) and extensive amount of plaque (P4). A small focus of “orphan” calcium along the distal LCX supports the diagnosis of chronic total occlusion.
3.1.1. CAD-RADS categories 3, 4 and 5 require further consideration—In the presence of CAD-RADS 3, which reflects moderate stenosis (50–69%), there is an option to consider the use of CT-FFR, CTP, or stress testing (ETT, stress echocardiogram, SPECT, PET or Cardiac MRI) to document or exclude the presence of ischemia. Further testing should be considered if this information will change patient management and, in the presence of stable or acute chest pain, if the patient has persistent symptoms despite adequate medical therapy. In addition to symptoms, it is important to consider other factors, such as stenosis lesion location and severity, and the presence of high-risk plaque features. Ultimately, the need for invasive coronary angiography as the next step in patient management requires careful integration of all clinical data together with all available imaging and stress test findings.
For CAD-RADS 4, recommendations may vary depending on the involvement of left main coronary artery and the presence of severe obstructive three-vessel disease (>70%). If a left main coronary artery stenosis at least greater than 50% is suspected or if the examination demonstrates three-vessel obstructive disease, then further evaluation with invasive angiography and possible revascularization is recommended. For this reason, CAD RADS 4 is sub-divided into A and B:
CAD RADS 4A - This category indicates the case of a single vessel or two vessels demonstrating severe stenosis (70–99%). Further evaluation with ICA or functional imaging, including CT-FFR, CTP and stress testing (ETT, stress echocardiogram, SPECT, PET or Cardiac MRI) or invasive FFR is usually recommended depending on location, extent and severity of the lesion(s), and clinical characteristics, such as angina severity and the use of current guideline-directed medical therapies. It should be clarified that the benefit of revascularization is confined to patients with frequent symptoms despite optimal medical therapy. Other important considerations such as the presence of very-high-grade coronary stenosis (>90%) or high-risk plaque features as well as evidence of lesion-specific ischemia by FFR-CT or perfusion defects by myocardial CTP may favor the use of ICA as the next step in patient care, if revascularization is being considered. Persistent anginal symptoms despite medical therapy should also favor the use of ICA.
CAD RADS 4B - This indicates the presence of a left main stenosis of at least 50% or three-vessel obstructive disease (>70%). Further evaluation with ICA and possible revascularization is usually recommended, particularly for patients with frequent symptoms despite optimal medical therapy.
The clinical relevance of CAD-RADS 5 (total coronary occlusion) varies widely depending on the clinical context. It may be acute or chronic, and, in the context of chronic occlusion, factors such as lesion length, calcification particularly at the proximal aspect, tortuosity and degree of collateralization may be of relevance for management decisions (Fig 11).
Figure 11:
CAD-RADS N/P2. Motion artifacts obscuring the left main, LAD and LCX arteries, which renders these segments non-diagnostic (left) and moderate amount of plaque (P2 - Calcium Score = 247). Motion artifacts in the mid RCA (right) with calcified plaque.
Overall, a similar framework (Table 5) is used for patients with acute chest pain with other considerations including persistent clinical symptoms, troponin levels, EKG changes and high-risk plaque features leading to hospital admission and cardiology consultation for further work-up and management.
3.2. Plaque burden sub-classification
3.2.1. Overall amount of coronary plaque (“P”)—There are substantial data demonstrating that the overall amount of coronary plaque by CCTA has strong association with incident coronary heart disease events (36–40) and such information may offer stronger prognostic value than merely the presence or absence of anatomical stenosis and clinical variables (41). Indeed, the ability to detect the presence and amount of plaque by CCTA is a unique attribute of cardiac CT when compared with other non-invasive tests.
The updated version of CAD-RADS classification incorporates the designation “P” with categories ranging from P1 to P4 to categorize the overall amount of plaque as mild, moderate, severe or extensive on a per-patient basis (Table 2). Please note that CAD-RADS 0 denotes absence of stenosis or plaque, therefore P0 is not required as a classification. Importantly, there is currently no single method that is used to quantify the overall amount of plaque and thus the CAD-RADS classification enables imagers to select the technique which is most relevant for each CCTA study at a given institution. Assessment of plaque burden within an individual patient may vary substantially depending upon the method applied. Thus, it is recommended that imagers select the technique which is considered most appropriate for the individual patient and according to local practice norms. However, it is important to emphasize that when multiple different approaches can be performed to assess plaque burden, the most severe plaque assessment for the study should be used. It is also important to highlight that the P recommendations based on CAC or SIS is supported by prior evidence (36) and may be more reproducible. The methods for reporting total coronary plaque burden include the following:
CAC testing - CAC provides a reproducible, and accurate method to quantify the amount of calcified plaque burden. The total CAC score is an established surrogate of overall coronary plaque burden. When performed as part of a CCTA exam, CAC testing (most commonly quantified according to the Agatston method) can be used to identify the overall amount of plaque (Table 2). However, calcium score should not be used in isolation and should be combined with at least a qualitative assessment of total plaque burden (calcified and non-calcified) to ensure that non-calcified plaque is also accounted. Therefore, the plaque burden and “P” category based on Calcium score will stay the same (if no non-calcified plaque is seen) or may increase after incorporating information on the total burden of non-calcified plaque. Moreover, institutional protocols may not always include CAC testing as a component of CCTA and importantly, the CAC score alone lacks the important quantification of non-calcified plaque burden.
Segment involvement score (SIS) - the segment involvement score can easily be calculated from CCTA by assigning a score of 1 for each of the 16 coronary segments with any detectable plaque (highest possible score = 16) (36). This method provides an estimate of the overall extent of coronary plaque, and there are several studies demonstrating that a larger SIS is associated with higher rates of cardiovascular death or MI (38,41).
Visual estimate of overall plaque burden - this method is based on a qualitative estimate of the amount of calcified and non-calcified plaque in each coronary vessel, and then providing an assessment of overall plaque burden (Table 2; Examples Figs 2–6).
Quantitative Assessment of Total Coronary Plaque - The writing group discussed various quantitative approaches that are available to quantify total coronary plaque volume on CCTA (42). While there are numerous important emerging techniques for performing a quantitative and reproducible assessment of total plaque burden and plaque type beyond visual assessment alone, these techniques are not widely available and not routinely performed as part of clinical CCTA interpretation. In addition, most of the available techniques are time and labor intensive, which inhibit incorporation into routine clinical interpretation. These techniques require further validation against other techniques, including intravascular ultrasound, optical coherence tomography, and histology. In addition, clinical registries and multi-center trials will need to validate the reproducibility of different approaches, as well as establish sex and age reference ranges that can enhance risk assessment. The writing group anticipates that future iterations of CAD-RADS will incorporate novel techniques for plaque quantification as these become more developed and widely used.
The writing group recognizes that providing various different options to estimate overall plaque burden may lower the reproducibility of such an assessment. However, it is important to offer different options in recognition that different methods are used by different centers. Moreover, providing flexible options to estimate the overall amount of plaque is important to facilitate routine assessment of plaque burden as part of the clinical reading. Ultimately, gaining wider adoption by CCTA programs to report the overall amount of plaque may be more important than reliance upon a particular technique. Moreover, the CAD-RADS recommendations for patient management using plaque assessment are mostly based on expert opinion (i.e. the number of studies that have evaluated the efficacy of various therapies based on different thresholds of atherosclerosis is limited). As such, there are no absolute consensus thresholds based on these categories, but rather a framework whereby more aggressive therapies are suggested for individuals that have a higher plaque burden (See Tables 2, 4 and 5).
3.3. Modifiers
CAD-RADS categories can be complemented by modifiers to indicate that a study is not fully evaluable or non-diagnostic (N), or to indicate the presence of stents (S), grafts (G), and high-risk plaque (HRP). In this updated CAD-RADS version, the panel has added two new modifiers: ischemia (I) and exceptions (E). In addition, the term “vulnerable plaque (V)” has now been replaced with “high risk plaque (HRP)” to be consistent with evolving terminology.
3.3.1. Modifier N – non-diagnostic study— “N” can be used as a modifier or as a CAD-RADS category, depending on context. If the study is not fully diagnostic, due to motion artifacts, calcium blooming, metal artifacts or other types of artifacts, (i.e. not all segments >1.5 mm diameter can be interpreted with confidence) and a stenosis ≥50% is present in a diagnostic segment (CAD-RADS ≥ 3), the most severe stenosis should be graded in addition to the modifier N. For example, a patient with moderate stenosis (50–69%) in one segment and one or more non-diagnostic segments and also moderate amount of plaque burden, should be graded as CAD-RADS 3/P2/N (Fig 12) and not CAD-RADS N, since further evaluation is needed, possibly with functional imaging, and patient recommendations for anti-ischemic and preventive management apply. However, for a patient with at least one non-interpretable segment and no stenosis (zero), minimal (1–24%), or no more than mild stenosis (25–49%) in interpretable segments, CAD- RADS N should be used since CCTA cannot reliably exclude a significant stenosis and cannot be used to guide patient management and hence, further evaluation is still needed. Category “P” should be used with category or modifier “N”, if total coronary plaque assessment can be performed reliably. Category “N” should precede Category “P” in replacement of the numerical stenosis assessment, if there is a non-interpretable coronary segment and no other coronary segment with greater than 50% stenosis. On the other hand, the numerical stenosis category and category “P” will precede Modifier “N” if there is a stenosis greater than 50% (CAD-RADS 3 or greater).
3.3.2. Modifier S = stent - presence of coronary stents—The modifier “S” indicates the presence of at least one coronary stent anywhere in the coronary system. For example, if a patient has a stent in the proximal left anterior descending coronary artery (LAD) with no significant in-stent restenosis or occlusion and demonstrates mild non-obstructive disease (25–49%) in the left circumflex (LCX) and right coronary arteries (RCA), the CCTA would be classified as: CAD-RADS 2/S. If a patient demonstrates significant in-stent restenosis of a stent in the proximal LAD, the study would be classified as: CAD-RADS 4A/S (Fig 13). Similarly, a non-stenotic stent in the LAD and a new severe stenosis in the RCA would be classified as CAD-RADS 4A/S. Finally, if a stent is non-evaluable, the study would be classified as CAD-RADS N/S if there is no other stenosis greater than 50% in the coronary tree. Note: CAD-RADS was created to guide management recommendations, so it does not matter whether the severe stenosis is in stented or non-stented vessel. Rather, the key issue is whether the patient has a severe stenosis and may be considered for further work-up. Category P should also be added to indicate the amount of plaque burden.
Figure 13:
CAD-RADS 4A/P3/S. In-stent stenosis of the proximal LAD with significant luminal narrowing (70–99% stenosis) and severe amount of coronary plaque (P3). Grading of in-stent stenosis should follow the grading of normal coronary arteries (0% stenosis, 1–24% stenosis, 25–49% stenosis, 50–69% stenosis, 70–99% stenosis, and >99% stenosis). In this case, severe in-stent restenosis designates a CAD-RADS 4A lesion, which would be followed by category P3 for extensive plaque burden and the stent modifier “S” for the presence of stent.
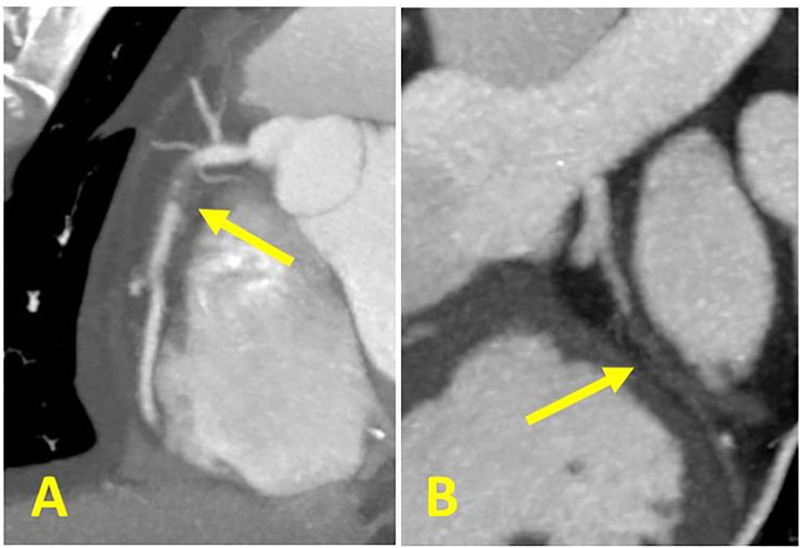
3.3.3. Modifier G = grafts - presence of coronary bypass grafts—The modifier “G” indicates the presence of at least one coronary-artery bypass graft (Fig 14). A stenosis bypassed by a fully patent graft is not considered for the CAD-RADS classification. For example, if a patient has a graft to LAD, with absence of significant stenosis in the graft, distal anastomosis and run-off vessel, and demonstrates non-obstructive lesions (25–49%) in the LCX and RCA, in addition to the “expected” proximal LAD severe stenosis, and moderate plaque burden, the case would be classified as: CAD-RADS 2/P2/G. In the example of a patient with total occlusion of a saphenous vein graft (SVG) to the RCA, and a patent LIMA to LAD and SVG to LCX, and severe plaque burden, the case would be classified as: CAD-RADS 5/P3/G. The interpretation is that a total occlusion is present and further management or investigation may be considered. Total plaque burden should be assessed in both native coronary arteries and by-pass grafts. A combined assessment should be considered for deciding on Category “P”.
Figure 14:
MODIFIER G. Coronary CTA demonstrating a patent left internal mammary artery to the LAD and patent saphenous vein grafts to the ramus intermedius and second obtuse marginal branch. No stenoses or luminal narrowing throughout the grafts (0% stenosis, left). Invasive coronary angiography demonstrating patent LIMA graft to the LAD (right). When evaluating coronary CTA of patients with bypass grafts, the native coronary artery segments proximal to the graft anastamoses should not be evaluated for purposes of CAD RADS coding. Only the grafts and the native coronary artery segments distal to and including the anastomosis should be evaluated for CAD RADS coding.
3.3.4. High-risk plaque (HRP) features (previously “vulnerable plaque” [V])—Data from recent CCTA studies have described high-risk plaque characteristics that are associated with a higher risk of future ACS as well as lesion specific ischemia. Features originally described as indicating HRP include positive remodeling, low-attenuation plaque, spotty calcification, and the napkin-ring sign (43–46). These plaque characteristics are associated with intravascular ultrasound and histological features of more advanced atherosclerotic plaque and thin cap fibroatheroma, which has the potential to develop to plaque rupture/thrombosis. However, the prevalence of these features on CCTA is high (~30% of CCTA, with an even higher frequency in the presence of stenosis), and thus the positive predictive value for identifying future events is relatively modest, especially when evaluated on top of plaque burden (39).
If a coronary plaque clearly demonstrates two or more high-risk features by CCTA, the modifier “HRP” (high risk plaque) should be added (Fig 15 and 16). High-risk features include: spotty calcifications, low attenuation plaque (less than 30 Hounsfield units), positive remodeling, and the “napkin ring sign” (see Fig 15).
Figure 15:
![High-risk plaque (HRP) features on coronary CTA. (A) Spotty calcium, defined as punctate calcium within a plaque (B) “napkin ring sign,” defined in a non-calcified plaque cross-sectional image by the presence of two features: a central area of low attenuation plaque that is apparently in contact with the lumen; and a ring-like peripheral rim of higher CT attenuation surrounding this central area (arrows); (C) Positive remodeling, defined as the ratio of outer vessel diameter at the site of plaque divided by the average outer diameter of the proximal and distal vessel greater than 1.1, or Av/[(Ap + Ad)/2] >1.1; and (D) Low attenuation plaque, defined as non-calcified plaque with internal attenuation less than 30 HU. Please note that a combination of two or more high-risk features is necessary to designate the plaque as high-risk for CAD-RADS.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ae9d/9627235/c0ab3a069aab/ryct.220183.fig15.jpg)
High-risk plaque (HRP) features on coronary CTA. (A) Spotty calcium, defined as punctate calcium within a plaque (B) “napkin ring sign,” defined in a non-calcified plaque cross-sectional image by the presence of two features: a central area of low attenuation plaque that is apparently in contact with the lumen; and a ring-like peripheral rim of higher CT attenuation surrounding this central area (arrows); (C) Positive remodeling, defined as the ratio of outer vessel diameter at the site of plaque divided by the average outer diameter of the proximal and distal vessel greater than 1.1, or Av/[(Ap + Ad)/2] >1.1; and (D) Low attenuation plaque, defined as non-calcified plaque with internal attenuation less than 30 HU. Please note that a combination of two or more high-risk features is necessary to designate the plaque as high-risk for CAD-RADS.
Figure 16:
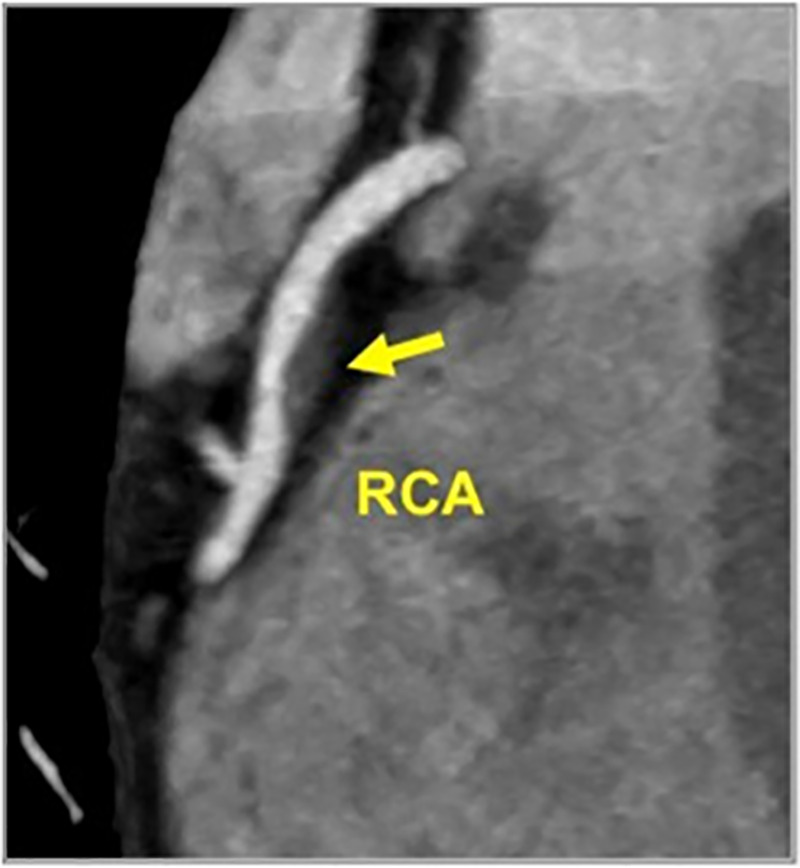
CAD-RADS 2/P2/HRP. Focal non-calcified plaque in the mid RCA with 25–49% diameter stenosis and overall moderate amount of total coronary plaque. The plaque demonstrates two high risk features, low attenuation (<30 HU) and positive remodeling, thus coding with the modifier “HRP.”
As an example, HRP should be used for a patient demonstrating plaque with two or more high-risk features (spotty calcifications, positive remodeling, low attenuation plaque or the napkin ring sign) (Fig 16). The specific features should be described in the report text.
When deciding on how the presence of HRP should impact patient management it is important to recognize that these features have been associated with (a) acute chest pain – a higher risk of ACS, independent of stenosis severity (44); (b) stable chest pain – a higher risk of incident adverse cardiovascular events (39,47); (c) a higher likelihood of lesion specific ischemia, as defined by invasive FFR. Therefore, among patients with acute chest pain who have HRP, hospital admission or observation may be considered even in the absence of severe stenosis. If the patient is discharged from an acute chest pain presentation, short-term clinical follow-up may be useful. Among patients with stable chest pain, the presence of HRP may be most relevant in the presence of non-obstructive CAD or when there is uncertainty regarding whether lesion specific ischemia is present. Importantly, regardless of the clinical setting (i.e. acute or stable chest pain), the identification of HRP (i.e. 2 or more features) – similar to the presence of more extensive plaque, should signify the need for more aggressive preventive therapies (i.e. statins and possibly aspirin), including for nonobstructive lesions (CAD-RADS 1 and CAD-RADS 2).
Studies coded with CAD-RADS 3 and HRP (the presence of high risk plaque with 50–69% diameter stenosis, excluding left main lesions) should prompt consideration of more aggressive management than studies coded only with CAD-RADS 3, particularly in patients presenting to the emergency department with acute chest pain. This includes consideration of further testing with CT-FFR, CTP, other stress imaging, or invasive coronary angiography depending on the clinical symptoms, EKG findings and biomarkers. However, management decisions should ultimately be made on an individual basis taking into consideration all supporting clinical and laboratory data.
3.3.5. Modifier I = ischemia: CT-FFR or CTP—Historically, CCTA exclusively provided anatomical information comprising luminal stenosis severity and atherosclerotic burden. Given the growing evidence regarding the critical importance of physiology to guide decisions around coronary revascularization and the development of techniques enabling functional assessment of CCTA the writing group deemed it important to update the CAD-RADS reporting guidelines to reflect this practice shift. The Modifier “I” indicates that an ischemia test has been performed (either CT-FFR or stress CTP).
3.3.5.1. Computed tomography fractional-flow reserve (CT-FFR)—CT-FFR was first introduced over a decade ago and allows for the computation of pressure across the coronary tree through the integration of machine learning for anatomical data extraction and computational fluid dynamics. The technique has been shown to be accurate and demonstrates excellent agreement with invasive FFR (48). There is also growing clinical utility data across multiple healthcare systems documenting the safety of deferral from catheterization in the setting of a negative CT-FFR (>0.80), the improved catheterization lab efficiency (ICA) and increased risk associated with an CT-FFR ≤0.80 (49). There is also growing evidence of the continuous rather than discrete nature of physiology with increasing risk with lower CT-FFR values. Given the non-binary nature of CT-FFR, current clinical guidance emphasizes that the CT-FFR value 1–2 cm distal to an area of coronary stenosis should be considered to guide decisions around referral to invasive angiographic and revascularization (50). The lowest overall value along the entire vessel may be informative but typically reflects total plaque burden and the ratio of coronary volume to mass, and hence reflects vascular health, as a result it can be used to inform medical management but should not be used to guide catheterization laboratory referral (51).
To that end, current evidence suggests possible ICA referral for a symptomatic patient in the setting of an appropriate clinical context for coronary revascularization and the designation of “I+” (positive ischemia) for a lesion-specific value ≤ 0.75 in a vessel large enough for percutaneous coronary intervention (PCI). Similarly, deferral of ICA would be approxi-mate in the setting of a “I−” (negative ischemia) lesion-specific CT-FFR >0.80 (Table 6). For values between 0.76 and 0.80 the modifier “I+/−” (borderline or indeterminate value) is used and decisions around ICA referral will further depend on lesion location, symptom severity and delta CT-FFR (trans-lesional gradient >0.12 considered significant) as measured as the pressure loss from 1 to 2 cm proximal to 1–2 cm distal to a stenosis (52). For lesions with an abnormal CT-FFR without a concordant anatomic lesion, the modifier “I−” should be described in case the reader is confident that this is false-positive result by CT-FFR or “I+/−” if it is indeterminate and there is questionable interpretation of both findings. In multivessel disease the physiologically significant lesion may not be the most anatomically severe. This needs to be contextualized and clarified in the body of the report and impression. In case the CT-FFR study is non-diagnostic, modifier N can also be applied to CT-FFR or CTP. Table 6 summarizes interpretation of CT-FFR.
Table 6:
Interpretation of CT-FFR. If there is presence of abnormal CT-FFR as defined as a lesion-specific value ≤ 0.75 in a vessel large enough for PCI the designation of “I+” (I=Ischemia) should be included. “I-” in the setting of a lesion-specific CT-FFR >0.80. For values between 0.76 and 0.80 the modifier “I+/-” is used and decisions around ICA referral depend on lesion location, symptom severity and delta CT-FFR.
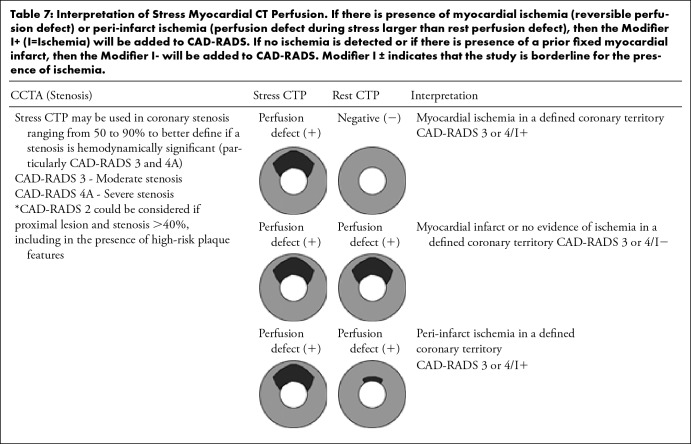
3.3.5.2. Myocardial CT perfusion—Advancements in CT technology, in particular the development of wide-area detectors with greater z-axis coverage and dual-source CT, have improved functional assessment of the myocardium using stress CTP. Myocardial CTP has been validated in patients with acute and chronic chest pain against modalities such as cardiac MRI, SPECT-MPI, invasive coronary angiography, invasive FFR and cardiac biomarkers (53–55). Furthermore, a combined approach of myocardial CTP with coronary CTA has been shown to have better diagnostic accuracy than CCTA alone in patients at intermediate-to-high risk for coronary artery disease (CAD) (56–58). Therefore, the Society of Cardiovascular Computed Tomography has provided resources to facilitate clinical implementation of CTP (59). The addition of stress myocardial CTP to CCTA allows detection of hemodynamically significant stenosis in a single setting with the identification of reversible myocardial ischemia correlating with the same territory in which a moderate or severe stenosis is suspected. Stress myocardial CTP also allows the exclusion of myocardial ischemia in moderate coronary stenosis (50–69%) or a suspected severe coronary stenosis (>70%) with dense calcified or mixed plaques, avoiding additional downstream testing. It also allows the identification of fixed perfusion defects related to prior myocardial infarction. Table 7 describes the interpretation of stress CTP and how it is incorporated with the different CAD-RADS categories. In the presence of myocardial ischemia (reversible perfusion defect) or peri-infarct ischemia (perfusion defect during stress larger than rest perfusion defect), the Modifier “I+” should be added to CAD-RADS. If no ischemia is detected or if there is presence of a prior fixed myocardial infarct, the Modifier “I−” will be added to CAD-RADS. The presence of myocardial infarct should be documented in the impression of the report and the Modifier “I” should be reserved exclusively to ischemia. The Modifier “I+/-” indicates that the study is borderline or inconclusive for the presence of ischemia. Similarly to the mismatch between CT-FFR and CCTA results, an ischemic segment without a concordant anatomic lesion, should be classified as modifier “I-” in case the reader is confident that this is a false-positive result by CTP or “I+/−” if it is indeterminate and there is questionable and discrepant interpretation of both findings. Either CT-FFR or CTP can be performed at the time of the CCTA interpretation or later. If performed later, the recommendation is to update the CAD-RADS score by adding the Modifier “I”. Table 7 summarizes interpretation of myocardial CTP.
Table 7:
Interpretation of Stress Myocardial CT Perfusion. If there is presence of myocardial ischemia (reversible perfusion defect) or peri-infarct ischemia (perfusion defect during stress larger than rest perfusion defect), then the Modifier I+ (I=Ischemia) will be added to CAD-RADS. If no ischemia is detected or if there is presence of a prior fixed myocardial infarct, then the Modifier I- will be added to CAD-RADS. Modifier I ± indicates that the study is borderline for the presence of ischemia.
3.3.6. Modifier E = exceptions—In clinical practice, sites that have adopted the CAD-RADS classification report scores approximately 95% of the time for CCTA (14). In general, CAD-RADS scores are not used in cases of non-atherosclerotic causes of coronary abnormalities, such as coronary dissections, anomlous coronary arteries, coronary artery aneurysms or pseudo-aneurysms, vasculitis, coronary artery fistulas, extrinsic coronary artery compression and other causes (Table 3). These exceptions are far less frequent than atherosclerosis as a cause of coronary stenosis or obstruction but remain important differential diagnostic considerations and are increasingly being recognized. Therefore, in the updated version of CAD-RADS, a modifier “E” is used to account for any non-atherosclerotic narrowing of the coronary arteries and should be added at the end of the score as a modifier. For example, if an anomalous coronary artery with inter-arterial course results to a moderate stenosis, then CAD-RADS 3/E should be coded. The modifier “E” will have the following purposes: 1- it will allow for non-atherosclerotic causes of coronary obstruction to be identifiable in the CAD-RADS reporting system; 2- it will provide a framework for tracking of such etiologies; 3- and it will indicate to the referring clinician that the CAD-RADS classification, which is strictly related to atherosclerotic coronary artery disease, may not fully capture the full range of coronary abnormalities.
VII. The framework for the new CAD-RADS coding should follow three categories: stenosis, plaque and then modifiers. Therefore, the Category “P” for plaque should follow the CAD-RADS score for stenosis. Then modifiers should be added, if present. If more than one category and/or modifier is present, the symbol “/” (slash) should follow each modifier in the following order:
First: modifier N (non-diagnostic)
Second: modifier HRP (high-risk plaque)
Third: modifier I (ischemia)
Fourth: modifier S (stent)
Fifth: modifier G (graft)
Sixth: modifier E (exceptions)
Examples:
Non-interpretable coronary stent with moderate amount of plaque burden without evidence of other obstructive coronary disease: Categories N and P should be used and Modifier S = CAD- RADS N/P2/S. Please note that Category “N” will replace the numerical stenosis grading and will precede Category “P”
Presence of a stent and at least one moderate stenosis demonstrating severe amount of plaque burden and high-risk plaque features: Modifiers S and HRP = CAD-RADS 3/P3/HRP/S (Fig 17)
Presence of stent, grafts, severe amount of plaque burden and non-evaluable segments due to metal artifacts: Categories N and P and Modifiers S and G = CAD-RADS N/P3/S/G
Presence of a patent LIMA graft to the LAD and expected occlusion of the proximal LAD and extensive amount of plaque burden in the native coronary arteries. Mild non-obstructive stenosis in the RCA and LCX. Modifier G = CAD-RADS 2/P4/G
For a patient with severe stenosis (70–99%) in one segment with severe amount of plaque burden and a non-diagnostic area in another segment, the study should be graded as CAD-RADS 4A/ P3/N
Presence of moderate stenosis (50–69%) with severe amount of plaque burden and FFR-CT performed with a value < 0.75. CAD-RADS 3/P3/I+
Presence of severe stenosis in the distal RCA (70–99%) with moderate amount of plaque burden and stress CTP demonstrating no evidence of reversible ischemia. CAD-RADS 4A/P2/I-
Anomalous left main coronary artery from the right sinus of Valsalva with inter-arterial course leading to severe compression and stenosis, absence of coronary plaque and positive stress CTP. CAD-RADS 4A/I+/E - Please note that because there is no evidence of plaque, the category “P” is not used
Figure 17:
CAD-RADS 3/P3/HRP/S. Example demonstrating a patent stent (S) in the proximal RCA (0% stenosis) with high-risk plaque (HRP) in the proximal LAD with thick MIP images resulting in 50–69% stenosis and overall severe amount of total coronary plaque burden (P3). In isolation, the proximal LAD lesion would be coded CAD RADS 3/P3/HRP. However, since CAD RADS is coded on a per-patient basis, and a RCA stent is present, this patient would be coded as CAD RADS 3/P3/S/HRP.
3.4. Presence of other cardiac or extra-cardiac findings—
Patients undergoing CCTA may demonstrate other significant, potentially significant or non-significant cardiac or extra-cardiac findings. CAD-RADS is intended to focus solely on the classification of coronary artery stenosis and further management. However, other cardiac and extra-cardiac findings of relevance should be reported in the body and/or impression of the CCTA report. Specific follow-up and recommendations should be included depending on the pathology.
Table 8 summarizes the main changes for 2022 CAD-RADS update when compared to the first version published in 2016. Table 9 describes the potential sources of troponin elevation and Figure 18 provides a sample standardized reporting template for CCTA incorporating CAD-RADS coding. Table 10 summarizes suggested text for recommendations section of CCTA reporting in patients with stable chest pain and Table 11 summarizes suggested text for recommendations section of CCTA reporting in patients with acute chest pain.
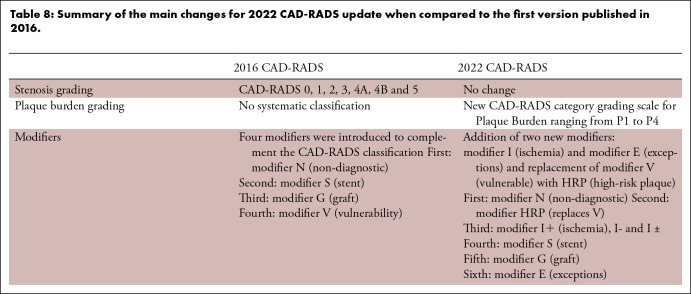
Table 8:
Summary of the main changes for 2022 CAD-RADS update when compared to the first version published in 2016.
Table 9:
Potential sources of high-sensitivity troponin elevation.a
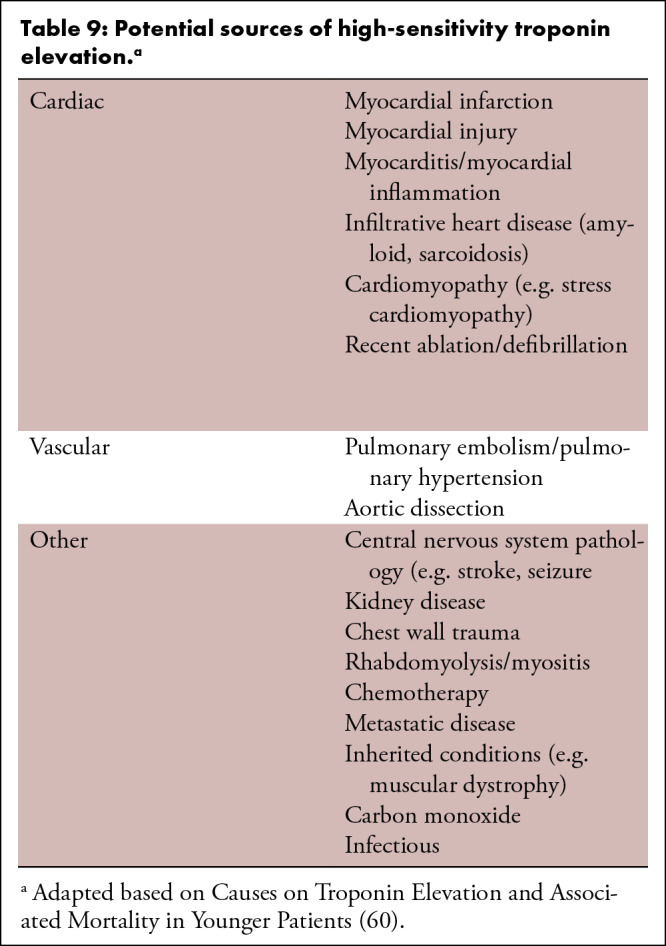
Figure 18:
Sample standardized reporting template for Coronary CTA incorporating CAD-RADS coding.
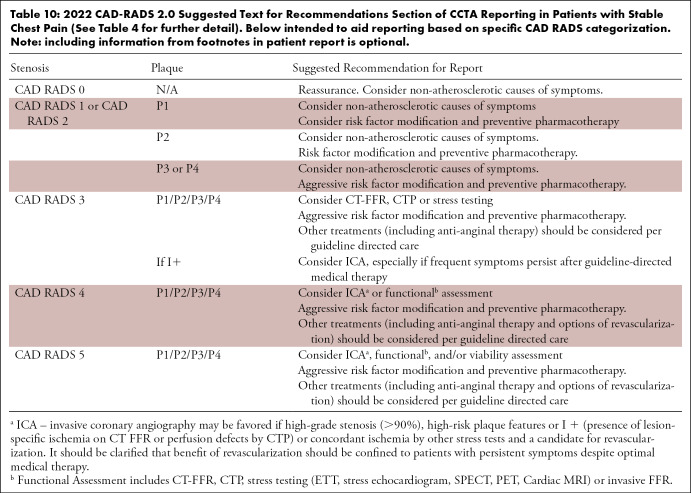
Table 10:
2022 CAD-RADS 2.0 Suggested Text for Recommendations Section of CCTA Reporting in Patients with Stable Chest Pain (See Table 4 for further detail). Below intended to aid reporting based on specific CAD RADS categorization. Note: including information from footnotes in patient report is optional.
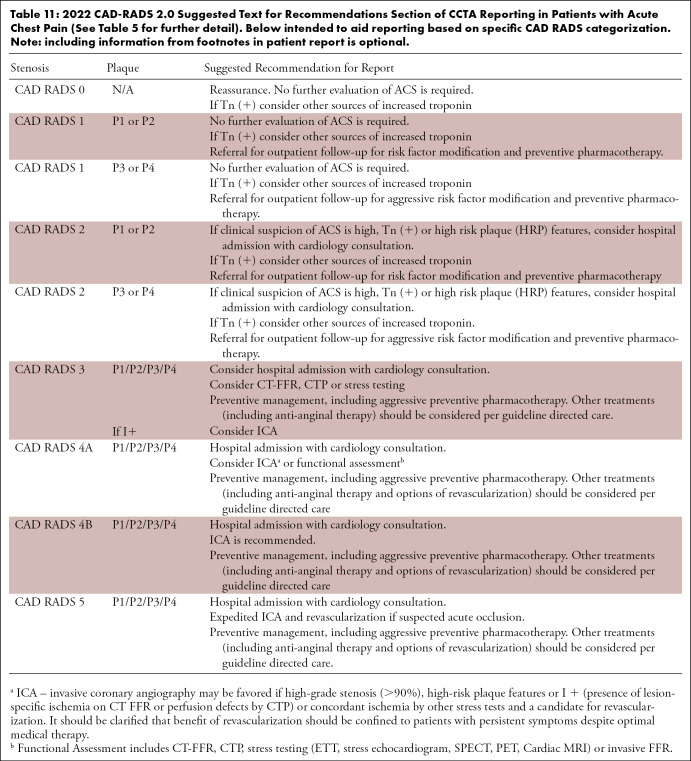
Table 11:
2022 CAD-RADS 2.0 Suggested Text for Recommendations Section of CCTA Reporting in Patients with Acute Chest Pain (See Table 5 for further detail). Below intended to aid reporting based on specific CAD RADS categorization. Note: including information from footnotes in patient report is optional.
MODIFIERS: The framework for the new CAD-RADS coding should follow: stenosis, plaque and then modifiers. Therefore, the Category “P” for plaque should follow the CAD-RADS score for stenosis. Then the modifier should be added. If more than one modifier or category is present, the symbol “/” (slash) should follow each category or modifier in the following order:
First: modifier N (non-diagnostic)
Second: modifier HRP (high-risk plaque)
Third: modifier I (ischemia)
Fourth: modifier S (stent)
Fifth: modifier G (graft)
Sixth: modifier E (exceptions)
Modifier E = Exceptions to CAD-RADS/non-atherosclerotic abnormalities. Modifier E should be used in addition to CAD-RADS 0–5. Non-atherosclerotic narrowing of the coronary arteries may require disease-specific management considerations and/or subspecialty referral.
MODIFIERS: The framework for the new CAD-RADS coding should follow: stenosis, plaque and then modifiers. Therefore, the Category “P” for plaque should follow the CAD-RADS score for stenosis. Then the modifier should be added. If more than one modifier or category is present, the symbol “/” (slash) should follow each category or modifier in the following order:
First: modifier N (non-diagnostic)
Second: modifier HRP (high-risk plaque)
Third: modifier I (ischemia)
Fourth: modifier S (stent)
Fifth: modifier G (graft)
Sixth: modifier E (exceptions)
Modifier E = Exceptions to CAD-RADS/non-atherosclerotic abnormalities. Modifier E should be used in addition to CAD-RADS 0–5. Non-atherosclerotic narrowing of the coronary arteries may require disease-specific management considerations and/or subspecialty referral.
4. Discussion
CAD-RADS has been developed based on scientific data, consensus guidance from cardiac imaging experts and a multi-disciplinary effort involving societies comprised of radiologists and cardiologists (SCCT, ACR, ACC and NASCI). It has been extensively validated over the past 5 years and has shown to provide a clinically useful categorization of coronary artery disease with high diagnostic accuracy when compared with invasive angiography. CAD-RADS has also shown to provide prognostic value and to impact medical management.
CAD-RADS is intended to be a “living document” that will undergo continuing development to provide up-to-date, evidence-based recommendations that allow imagers to communicate with providers and to convey concise findings using unambiguous and standardized terminology. Beyond its utilization in clinical reporting, CAD-RADS will allow reliable and reproducible data collection, storage and retrieval for future research trials and audits.
CAD-RADS has the potential to provide the basis as a framework for standardized collection of coronary CTA reports across multiple sites for quality improvement, benchmarking, registries and multi-center trials. Further, it can provide the framework for collecting outcome data in each of several sub-categories of CAD-RADS, such as:
Follow-up of patients based on CCTA results;
Rate of downstream testing;
Correlation with ICA;
Rate of coronary revascularization including percuta neous coronary intervention and coronary artery by pass graft surgery;
Major adverse cardiac events, including cardiovascular death and myocardial infarct.
Therefore, it is recommended that CCTA reporting use the CAD-RADS classification in addition and in conjunction with the impression of the report. The writing group recognizes that the CAD-RADS classification alone will not always provide all the information which is necessary to convey to referring providers for the purposes of patient management. Furthermore, the recommendations provided by this document may not apply to every clinical scenario. Therefore, imagers should provide additional comments, advice, and convey any degree of uncertainty whenever applicable.
Peer-reviewed journals may also find the CAD-RADS terminology useful for standardized classification of CCTA results, which in turn will further promote the use of CAD-RADS nationally and internationally.
Finally, standardization of reports and management recommendations will not only improve the clarity of communication of imaging results among all members of the clinical care team, but also will enhance communication between imagers, providers, researchers, and computer- based systems. This may facilitate the development of decision support technologies and serve as the basis for developing artificial intelligence and natural language processing algorithms.
5. Conclusion
The 2022 updated CAD-RADS version enhances the initial standardized reporting system for CCTA by including data from recent large trials, new clinical guidelines, and by adding features of plaque burden and lesion physiology determined from cardiac CT. Hence, the updated CAD-RADS classification follows a framework of stenosis, plaque burden and modifiers which now also include ischemia evaluation by CT-FFR or myocardial CT perfusion, when applicable. With these new iterations, CAD-RADS will continue to provide an important framework of stan- dardization that is expected to benefit education, research, peer-review, artificial intelligence development, quality assurance, with the ultimate goal of improving patient care.
Declaration of competing interest :*In accordance with SCCT policy, writing group members and reviewers are required to disclose relationships with industry; see Appendices 1 and 2 for detailed information.
Abbreviations:
- CAD
- coronary artery disease
- CAD-RADS
- Coronary Artery Disease Reporting and Data System
- CAC
- coronary artery calcium
- CCTA
- coronary CT angiography
- CT-FFR
- computed tomography fractional flow reserve
- CTP
- computed tomography perfusion
- HRP
- high-risk plaque
- ICA
- invasive coronary angiography
- PCI
- percutaneous coronary intervention
- SIS
- segment involvement score
References
- 1.Cury RC.. President's page: ten years of innovation in cardiac CT. J Cardiovasc Comput Tomogr. 2014 Jul-Aug;8(4):338–339. [DOI] [PubMed] [Google Scholar]
- 2.Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009 May-Jun;3(3):190–204. [DOI] [PubMed] [Google Scholar]
- 3.Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014 Sep-Oct;8(5):342–358. [DOI] [PubMed] [Google Scholar]
- 4. Taylor AJ , Cerqueira M , Hodgson JM , et al . ACCF/SCCT/ACR/AHA/ASE/ASNC/ NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of cardiology foundation appropriate use criteria task force, the society of cardiovascular computed tomography, the American College of radiology, the American heart association, the American society of echocardiography, the American society of nuclear cardiology, the north American society for cardiovascular imaging, the society for cardiovascular angiography and interventions, and the society for cardiovascular magnetic resonance . J Cardiovasc Comput Tomogr . 2010. Nov-Dec ; 4 ( 6 ): 407.e1, 33 . [DOI] [PubMed] [Google Scholar]
- 5. White RD , Patel MR , Abbara S , et al . American College of Radiology; American College of Cardiology Foundation. ACCF/ACR/ASE/ASNC/SCCT/SCMR appropriate utilization of cardiovascular imaging in heart failure: an executive summary: a joint report of the ACR Appropriateness Criteria ® Committee and the ACCF Appropriate Use Criteria Task Force . J Am Coll Radiol . 2013. ; 10 ( 7 ): 493 – 500 . [DOI] [PubMed] [Google Scholar]
- 6. Wolk MJ , Bailey SR , Doherty JU , et al . American College of cardiology foundation appropriate use Criteria task force. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/ SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of cardiology foundation appropriate use Criteria task force, American heart association, American society of echocardiography, American society of nuclear cardiology, heart failure society of America, heart rhythm society, society for cardiovascular angiography and interventions, society of cardiovascular computed tomography, society for cardiovascular magnetic resonance, and society of thoracic surgeons . J Am Coll Cardiol . 2014. Feb 4 ; 63 ( 4 ): 380 – 406 . [DOI] [PubMed] [Google Scholar]
- 7. Rybicki FJ , Udelson JE , Peacock WF , et al . Emergency department patients with chest pain writing panel, emergency department patients with chest pain rating panel; appropriate utilization of cardiovascular imaging oversight committee. 2015 ACR/ACC/AHA/AATS/ACEP/ASNC/NASCI/SAEM/SCCT/SCMR/SCPC/ SNMMI/STR/STS appropriate utilization of cardiovascular imaging in emergency department patients with chest pain: a joint document of the American College of radiology appropriateness criteria committee and the American College of cardiology appropriate use criteria task force . J Am Coll Radiol . 2016. Feb ; 13 ( 2 ): e1 – e29 . 10.1016/j.jacr.2015.07.007. Epub 2016 Jan 22. PMID: 26810814 . [DOI] [PubMed] [Google Scholar]
- 8. Expert Panel on Cardiac Imaging , Shah AB , Kirsch J , Bolen MA , et al . ACR appropriateness Criteria® chronic chest pain-noncardiac etiology unlikely-low to intermediate probability of coronary artery disease . J Am Coll Radiol . 2018. Nov ; 15 ( 11S ): S283 – S290 . 10.1016/j.jacr.2018.09.021. PMID: 30392597 . [DOI] [PubMed] [Google Scholar]
- 9. Expert Panel on Cardiac Imaging , Batlle JC , Kirsch J , Bolen MA , et al . ACR appropriateness Criteria® chest pain-possible acute coronary syndrome . J Am Coll Radiol . 2020. May ; 17 ( 5S ): S55 – S69 . 10.1016/j.jacr.2020.01.027. PMID: 32370978. [DOI] [PubMed] [Google Scholar]
- 10. Kelion AD , Nicol ED . The rationale for the primacy of coronary CT angiography in the National Institute for Health and Care Excellence (NICE) guideline (CG95) for the investigation of chest pain of recent onset . J Cardiovasc Comput Tomogr . 2018. ; 12 : 516 – 522 . [DOI] [PubMed] [Google Scholar]
- 11. Knuuti J , Wijns W , Saraste A , et al . 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes . Eur Heart J. 2020. Jan 14 ; 41 ( 3 ): 407 – 477 . 10.1093/eurheartj/ehz425. Erratum in: Eur Heart J. 2020 Nov 21;41(44):4242. PMID: 31504439. [DOI] [PubMed] [Google Scholar]
- 12. Choi AD , Thomas DM , Lee J , et al . SCCT guideline for training cardiology and radiology trainees as independent practitioners (level II) and advanced practitioners (level III) in cardiovascular computed tomography: a statement from the society of cardiovascular computed tomography . Radiol Cardiothorac Imaging . 2020. ; 3 ( 1 ), e200480 . 10.1148/ryct.2020200480. PMID: 33778658; PMCID: PMC7978013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cury RC , Abbara S , Achenbach S , et al . CAD-RADS™ coronary artery disease – reporting and data system. An expert consensus document of the society of cardiovascular computed tomography (SCCT), the American College of radiology (ACR) and the north American society for cardiovascular imaging (NA . J Cardiovasc Comput Tomogr . 2016. ; 10 ( 4 ): 269 – 281 . [DOI] [PubMed] [Google Scholar]
- 14. Takigami AK , Thondapu V , Goiffon RJ , et al . Coronary artery disease reporting and data system (CAD-RADS) adoption: analysis of local trends in a large academic medical center . Radiol Cardiothorac Imaging . 2021. ; 3 ( 3 ), e210016 . 10.1148/ryct.2021210016. Published 2021 Jun 24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie JX , Cury RC , Leipsic J , et al . The coronary artery disease–reporting and data system (CAD-RADS): prognostic and clinical implications associated with standardized coronary computed tomography angiography reporting . JACC Cardiovasc Imaging . 2018. ; 11 ( 1 ): 78 – 89 . [DOI] [PubMed] [Google Scholar]
- 16. Williams MC , Moss A , Dweck M , et al . Standardized reporting systems for computed tomography coronary angiography and calcium scoring: a real-world validation of CAD-RADS and CAC-DRS in patients with stable chest pain . J Cardiovasc Comput Tomogr . 2020. ; 14 ( 1 ): 3 – 11 . [DOI] [PubMed] [Google Scholar]
- 17. Nam K , Hur J , Han K , et al . Prognostic value of coronary artery disease-reporting and data system (CAD-RADS) score for cardiovascular events in ischemic stroke . Atherosclerosis . 2019. ; 287 ( October 2018 ): 1 – 7 . [DOI] [PubMed] [Google Scholar]
- 18. Bittner DO , Mayrhofer T , Budoff M , et al . Prognostic value of coronary CTA in stable chest pain . JACC Cardiovasc Imaging . 2019. . 10.1016/j.jcmg.2019.09.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Popa LE , Petresc B , Catana C , et al . Association between cardiovascular risk factors and coronary artery disease assessed using CAD-RADS classification: a crosssectional study in Romanian population . BMJ Open . 2020. ; 10 ( 1 ): 1 – 7 . 10.1136/bmjopen-2019-031799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Basha MAA , Aly SA , Ismail AAA , Bahaaeldin HA , Shehata SM . The validity and applicability of CAD-RADS in the management of patients with coronary artery disease . Insights Imaging . 2019. ; 10 ( 1 ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodriguez-Granillo GA , Carrascosa P , Goldsmit A , Arbab-Zadeh A . Invasive coronary angiography findings across the CAD-RADS classification spectrum . Int J Cardiovasc Imag . 2019. ; 35 ( 11 ): 1955 – 1961 . 10.1007/s10554-019-01654-1 . [DOI] [PubMed] [Google Scholar]
- 22. Muacevic A , Adler JR , Boster J , et al . Adoption of the coronary artery disease reporting and data system: reduced downstream testing and cardiology referral rates in patients with non-obstructive coronary artery disease . Cureus . 2019. ; 11 ( 9 ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hull RA , Berger JM , Boster JM , et al . Adoption of coronary artery disease – reporting and Data System (CAD-RADSTM) and observed impact on medical therapy and systolic blood pressure control . J Cardiovasc Comput Tomogr . 2020. ;( January : 1–7 ). [DOI] [PubMed] [Google Scholar]
- 24. Muscogiuri G , Chiesa M , Trotta M , et al . Performance of a deep learning algorithm for the evaluation of CAD-RADS classification with CCTA . Atherosclerosis . 2020. ; 294 ( August 2019 ): 25 – 32 . 10.1016/j.atherosclerosis.2019.12.001 . [DOI] [PubMed] [Google Scholar]
- 25. Douglas PS , Hoffmann U , Patel MR , et al . PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease . N Engl J Med. 2015. Apr 2 ; 372 ( 14 ): 1291 – 1300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. SCOT-HEART investigators . CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial . Lancet . 2015. Mar 13 ;( 15 ): 60291 – 60294 . pii: S0140-6736. [DOI] [PubMed] [Google Scholar]
- 27. Goldstein JA , Chinnaiyan KM , Abidov A , et al . The CT-STAT (coronary computed tomographic angiography for systematic triage of acute chest pain patients to treatment) trial . J Am Coll Cardiol . 2011. ; 58 : 1414 – 1422 . [DOI] [PubMed] [Google Scholar]
- 28. Litt HI , Gatsonis C , Snyder B , et al . CT angiography for safe discharge of patients with possible acute coronary syndromes . N Engl J Med . 2012. ; 366 : 1393 – 1403 . [DOI] [PubMed] [Google Scholar]
- 29. Hoffmann U , Truong QA , Schoenfeld DA , et al . Coronary CT angiography versus standard evaluation in acute chest pain . N Engl J Med . 2012. ; 367 : 299 – 308 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamilton-Craig C , Fifoot A , Hansen M , et al . Diagnostic performance and cost of CT angiography versus stress ECG–a randomized prospective study of suspected acute coronary syndrome chest pain in the emergency department (CT-COMPARE) . Int J Cardiol . 2014. Dec 20 ; 177 ( 3 ): 867 – 873 . [DOI] [PubMed] [Google Scholar]
- 31. Cury RC , Feuchtner G , Battle J , et al . Triage of patients presenting with chest pain to the emergency department: implementation of coronary CTA in a large urban hospital healthcare system . Am J Roentgenol . 2013. . Jan ; 200 ( 1 ): 57 - 65 . [DOI] [PubMed] [Google Scholar]
- 32. Poon M , Cortegiano M , Abramowicz AJ , et al . Associations between routine coronary computed tomographic angiography and reduced unnecessary hospital admissions, length of stay, recidivism rates, and invasive coronary angiography in the emergency department triage of chest pain . J Am Coll Cardiol . 2013. ; 62 ( 6 ): 543 – 552 . [DOI] [PubMed] [Google Scholar]
- 33. Ghoshhajra BB , Takx RAP , Staziaki PV , et al . MGH Emergency Cardiac CTA Program Contributors. Clinical implementation of an emergency department coronary computed tomographic angiography protocol for triage of patients with suspected acute coronary syndrome . Eur Radiol . 2017. Jul ; 27 ( 7 ): 2784 – 2793 . 10.1007/s00330-016-4562-5. Epub 2016 Nov 24. PMID: 27885414; PMCID: PMC5976244 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raff GL , Chinnaiyan KM , Cury RC , et al . SCCT guidelines on the use of coronary computed tomographic angiography for patients presenting with acute chest pain to the emergency department: a Report of the Society of Cardiovascular Computed Tomography Guidelines Committee . J Cardiovasc Comput Tomogr . 2014. Jul-Aug ; 8 ( 4 ): 254 – 271 . [DOI] [PubMed] [Google Scholar]
- 35. Writing Committee Members , Gulati M , Levy PD , Mukherjee D , Amsterdam E , Bhatt DL , Birtcher KK , Blankstein R , Boyd J , Bullock-Palmer RP , Conejo T , Diercks DB , Gentile F , Greenwood JP , Hess EP , Hollenberg SM , Jaber WA , Jneid H , Joglar JA , Morrow DA , O'Connor RE , Ross MA , Shaw LJ . 2021 AHA/ACC/ASE/ CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines . J Cardiovasc Comput Tomogr . 2022. Jan-Feb ; 16 ( 1 ): 54 – 122 . 10.1016/j.jcct.2021.11.009. Epub 2021 Dec 1. PMID: 34955448. [DOI] [PubMed] [Google Scholar]
- 36. Shaw LJ , Blankstein R , Bax JJ , et al . Society of cardiovascular computed tomography/north American society of cardiovascular imaging - expert consensus document on coronary CT imaging of atherosclerotic plaque . J Cardiovasc Comput Tomogr . 2021. Mar-Apr ; 15 ( 2 ): 93 – 109 . 10.1016/j.jcct.2020.11.002. Epub 2020 Nov 9. PMID: 33303383. [DOI] [PubMed] [Google Scholar]
- 37. Adamson PD , Williams MC , Dweck MR , et al . Guiding therapy by coronary CT angiography improves outcomes in patients with stable chest pain . J Am Coll Cardiol . 2019. ; 74 : 2058 – 2070 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bittencourt MS , Hulten E , Ghoshhajra B , et al . Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events . Circulation Cardiovascular imaging . 2014. ; 7 : 282 – 291 . [DOI] [PubMed] [Google Scholar]
- 39. Williams MC , Moss AJ , Dweck M , et al . Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-heart study . J Am Coll Cardiol . 2019. ; 73 : 291 – 301 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mortensen MB , Dzaye O , Steffensen FH , et al . Impact of plaque burden versus stenosis on ischemic events in patients with coronary atherosclerosis . J Am Coll Cardiol . 2020. ; 76 : 2803 – 2813 . [DOI] [PubMed] [Google Scholar]
- 41. Lin FY , Shaw LJ , Dunning AM , et al . Mortality risk in symptomatic patients with nonobstructive coronary artery disease . A Prospective 2-Center Study of 2,583 Patients Undergoing 64-Detector Row Coronary Computed Tomographic Angiography . 2011. ; 58 : 510 – 519 . [DOI] [PubMed] [Google Scholar]
- 42. Williams MC , Earls JP , Hecht H . Quantitative assessment of atherosclerotic plaque, recent progress and current limitations , 1 J Cardiovasc Comput Tomogr . 2021. Jul 16 ; S1934 – 5925 ( 21 ): 398 . 10.1016/j.jcct.2021.07.001. Epub ahead of print. PMID: 34326003 . [DOI] [PubMed] [Google Scholar]
- 43. Motoyama S , Sarai M , Harigaya H , et al . Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome . J Am Coll Cardiol . 2009. ; 54 : 49 – 57 . [DOI] [PubMed] [Google Scholar]
- 44. Puchner SB , Liu T , Mayrhofer T , et al . High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial . J Am Coll Cardiol . 2014. ; 64 : 684 – 692 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maurovich-Horvat P , Hoffmann U , Vorpahl M , Nakano M , Virmani R , Alkadhi H . The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging . 2010. Apr ; 3 ( 4 ): 440 - 444 . [DOI] [PubMed] [Google Scholar]
- 46. Maurovich-Horvat P , Ferencik M , Voros S , Merkely B , Hoffmann U . Comprehensive plaque assessment by coronary CT angiography . Nat Rev Cardiol . 2014. Jul ; 11 ( 7 ): 390 – 402 . 10.1038/nrcardio.2014.60. Epub 2014 Apr 22. PMID: 24755916 . [DOI] [PubMed] [Google Scholar]
- 47. Ferencik M , Mayrhofer T , Bittner DO , et al . Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial . JAMA Cardiol . 2018. Feb 1 ; 3 ( 2 ): 144 – 152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nørgaard BL , Gaur S , Fairbairn TA , et al . Prognostic value of coronary computed tomography angiographic derived fractional flow reserve: a systematic review and meta-analysis . heartjnl-2021-319773 J.Heart . 2021. Oct 22 . 10.1136/heartjnl-2021-319773. Online ahead of print. PMID: 34686567 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patel MR , Nørgaard BL , Fairbairn TA , et al . 1-Year impact on medical practice and clinical outcomes of FFRCT: the ADVANCE registry . JACC Cardiovasc Imaging . 2020. Jan ; 13 ( 1 Pt 1 ): 97 – 105 . 10.1016/j.jcmg.2019.03.003. Epub 2019 Mar 17.PMID: 31005540. [DOI] [PubMed] [Google Scholar]
- 50. Takagi H , Leipsic JA , McNamara N , et al . Trans-lesional fractional flow reserve gradient as derived from coronary CT improves patient management: ADVANCE registry , 4 J Cardiovasc Comput Tomogr . 2021. Sep 2 ; S1934 – 5925 ( 21 ): 418 . 10.1016/j.jcct.2021.08.003. (Online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Driessen RS , Danad I , Stuijfzand WJ , et al . Comparison of coronary computed to- mography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis . J Am Coll Cardiol . 2019. Jan 22 ; 73 ( 2 ): 161 – 173 . 10.1016/j.jacc.2018.10.056. PMID: 30654888 . [DOI] [PubMed] [Google Scholar]
- 52. Takagi H , Leipsic JA , McNamara N , et al . Trans-lesional fractional flow reserve gradient as derived from coronary CT improves patient management: ADVANCE registry , 4 J Cardiovasc Comput Tomogr . 2021. Sep 2 ; S1934 – 5925 ( 21 ): 418 . 10.1016/j.jcct.2021.08.003. Epub ahead of print. PMID: 34518113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gonzalez JA , Lipinski MJ , Flors L , Shaw PW , Kramer CM , Salerno M . Meta-Analysis of diagnostic performance of coronary computed tomography angiography, computed tomography perfusion, and computed tomography-fractional flow reserve in functional myocardial ischemia assessment versus invasive fractional flow reserve . Am J Cardiol . 2015. ; 116 ( 9 ): 1469 – 1478 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang DH , Kim YH . CT myocardial perfusion imaging: current status and future perspectives . Int J Cardiovasc Imag . 2017. ; 33 ( 7 ): 1009 – 1020 . [DOI] [PubMed] [Google Scholar]
- 55. Ueki Y , Izawa A , Kashiwagi D , et al . Diagnostic advantage of stress computed tomography myocardial perfusion over single-photon emission computed tomography for the assessment of myocardial ischemia . J Cardiol . 2017. ; 70 ( 2 ): 147 – 154 . [DOI] [PubMed] [Google Scholar]
- 56. Pontone G , Baggiano A , Andreini D , et al . Diagnostic accuracy of simultaneous evaluation of coronary arteries and myocardial perfusion with single stress cardiac computed tomography acquisition compared to invasive coronary angiography plus invasive fractional flow reserve . Int J Cardiol . 2018. ; 273 : 263 – 268 . [DOI] [PubMed] [Google Scholar]
- 57. Pontone G , Baggiano A , Andreini D , et al . Dynamic stress computed tomography perfusion with a whole-heart coverage scanner in addition to coronary computed tomography angiography and fractional flow reserve computed tomography derived . JACC Cardiovasc Imaging . 2019. ; 12 ( 12 ): 2460 – 2471 . [DOI] [PubMed] [Google Scholar]
- 58. Yang J , Dou G , He B , et al . Stress myocardial blood flow ratio by dynamic CT perfusion identifies hemodynamically significant CAD . JACC Cardiovasc Imaging . 2020. Apr ; 13 ( 4 ): 966 – 976 . [DOI] [PubMed] [Google Scholar]
- 59. Patel AR , Bamberg F , Branch K , et al . Society of cardiovascular computed tomography expert consensus document on myocardial computed tomography perfusion imaging . J Cardiovasc Comput Tomogr . 2020. ; 14 ( 1 ): 87 – 100 . [DOI] [PubMed] [Google Scholar]
- 60. Wu C , Singh A , Collins B , et al . Causes of troponin elevation and associated mortality in young patients . Am J Med . 2018. Mar ; 131 ( 3 ): 284 – 292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fihn SD , Gardin JM , Abrams J , et al . ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of cardiology foundation/ American heart association task force on practice guidelines, and the American College of physicians, American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons . J Am Coll Cardiol . 2012. ; 60 ( 24 ): e44 – e164 . [DOI] [PubMed] [Google Scholar]
- 62. Arnett DK , Blumenthal RS , Albert MA , et al . ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of cardiology/ American heart association task force on clinical practice guidelines . Circulation . 2019. ; 140 ( 11 ): e596 – e646 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takigami AK , Thondapu V , Goiffon RJ , et al . Coronary artery disease reporting and data system (CAD-RADS) adoption: analysis of local trends in a large academic medical center . Radiol Cardiothorac Imaging . 2021. ; 3 ( 3 ), e210016 . Published 2021 Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]



![Timeline plots of total quarterly PubMed citations resulting from the search “CAD-RADS” [Title/Abstract] OR “CADRADS” [Title/Abstract]. The date of the search was January 25, 2021. Permission received (63). Radiol Cardiothorac Imaging. 2021 Jun; 3 (3): e210016.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ae9d/9627235/13b11d24ebfe/ryct.220183.fig1.jpg)