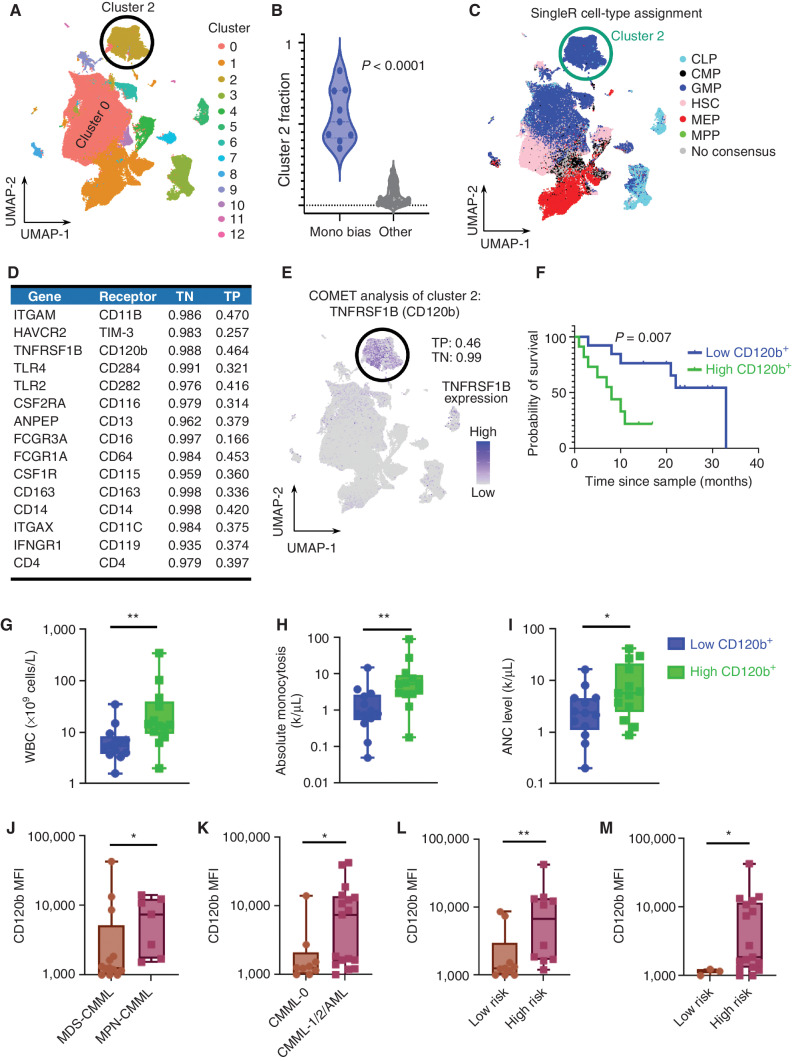
Figure 3.
Monocytic-biased CMML is associated with the expansion of an inflammatory GMP-like HSPC population. A, Graph-based clustering of the CD34+ cohort identified 13 distinct clusters across the 201,250 single cells. B, Samples in the monocytic-biased group were enriched in Clus2. C, SingleR was used to determine cell-type assignment using previously published paired references of bulk RNA-seq of flow cytometry–sorted cells. Clus2 was enriched for GMP cell-type assignment. D, COMET was used to identify differential gene-expression markers well-suited for validation with flow cytometry, and the top-ranked markers had an average log base 2-fold change of 4.50 in the single genes from Clus2 compared with the rest of the cells, average true positive rate of 36.7%, and average true negative rate of 98.0%. E, COMET identified TNFRSF1B (encoded cell-surface marker CD120b) as the best single marker for identifying Clus2 cells with a true positive performance of 46% and true negative performance of 99%. F, The predictive power of CD120b was validated in the complementary flow cytometry data set, where individuals with high CD120+ expression had inferior survival (n = 26; log-rank P = 0.007). Clinicopathologic variables were compared in CD120+ high vs. low patients and individuals with CD120+ high expression had (G) increased white blood cell (WBC) counts (P = 0.007), (H) increased absolute monocytosis (P = 0.004), and (I) increased absolute neutrophil count (ANC; P: 0.04). Patients with high CD120b GMPs were (J) myeloproliferative (P = 0.04), (K) associated with disease progression (P = 0.02), and (L) were categorized as high risk per MDACC (P = 0.005) and (M) Mayo prognostic scoring systems (P = 0.03), n = 26 patient cases. Nonparametric Mann–Whitney tests were used to compare two group data. P value significance represented by *, < 0.05; **, < 0.01; ***, < 0.001.