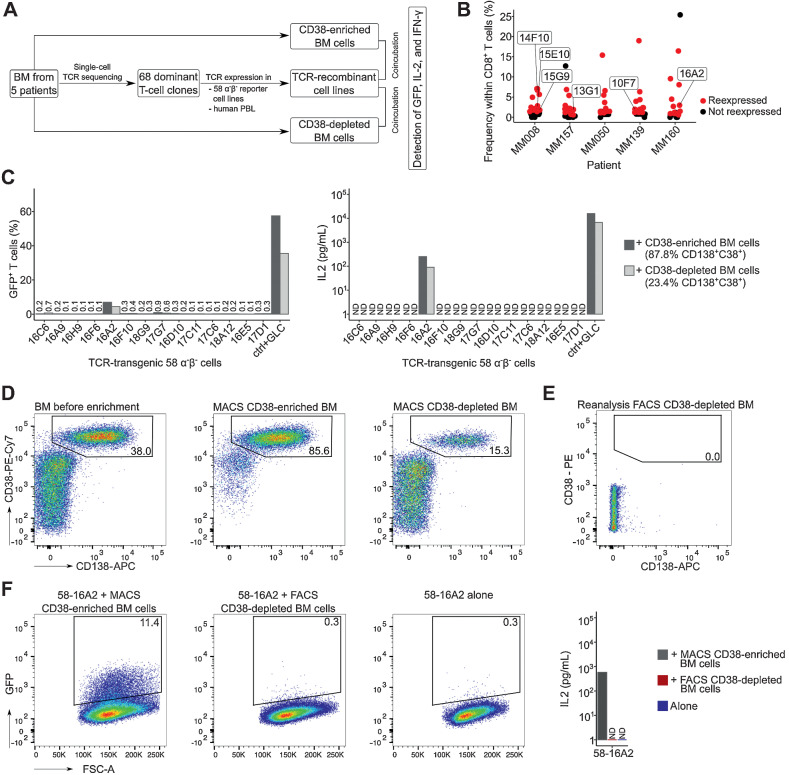
Figure 3.
Recognition of multiple myeloma cells by dominant bone marrow T-cell clones. A, Experimental setup: From five patients with substantial bone marrow T-cell expansion, 68 clones were selected for TCR expression in 58α−β− reporter T cells. TCR-recombinant cells were incubated with respective CD38-enriched multiple myeloma or CD38-depleted nonmyeloma cells for 16 hours at 37°C in 5% CO2 to determine antigen recognition. B, Patients and T-cell clones that were selected for expression (red) based on their frequencies. Data points indicate T-cell clones. Clones of mentioned in the manuscript are labeled. C, Incubation of TCR-recombinant 58α−β− reporter T cells with enriched multiple myeloma and nonmyeloma cells from patient MM160. 58α−β− cells expressing an HLA-A*02:01–restricted TCR specific for the EBV-derived peptide GLCTLVAML (GLC) were incubated for 16 hours at 37°C in 5% CO2 with myeloma and nonmyeloma cells from the same patient loaded with the target peptide as control (ctrl). GFP and murine IL2 were measured as readouts for T-cell activation. D, MACS CD38 enrichment and depletion of bone marrow from patient MM160. Cells were pregated on mononuclear cells. To remove remaining multiple myeloma cells (CD38++CD138+) from MACS CD38-depleted cells, (E) we performed subsequent FACS (CD19−CD38−CD138−, sorting gates in Supplementary Fig. S8), resulting in 0.0% remaining CD38++CD138+ multiple myeloma cells within the FACS CD38-depleted BM population. F, Repeated incubation of 58-16A2 with CD38-enriched and FACS CD38-depleted bone marrow from patient MM160 or 58-16A2 alone. Cells were pregated on single live murine CD3+ T cells after exclusion of human bone marrow cells by scatter characteristics, CD38, CD138, and CD45 staining. For each patient, incubations of reporter cells with CD38-enriched or depleted bone marrow cells were done in one experiment resulting in 5 experiments, unless stated otherwise. BM, bone marrow; PBL, peripheral blood lymphocytes; n.d., not detectable.