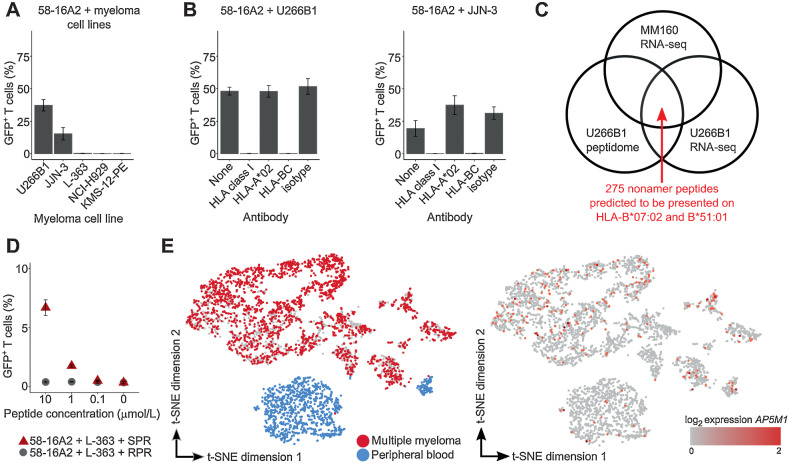
Figure 4.
TCR 16A2 recognizes the AP5M1-derived peptide SPRPPLISV (SPR). A and B, 3 × 104 58-16A2 were incubated with 5 × 104 cells of different multiple myeloma cell lines for 16 hours at 37°C 5% CO2. Blocking antibodies against HLA-class I, HLA-A*02, or HLA-BC were used at 20 μg/mL, 20 μg/mL, or 40 μg/mL, respectively. C, Potential target peptides of 16A2 were selected from a total of 3,686 nonamer peptides of the U266B1 peptidome applying the following criteria: (i) RNA expression within multiple myeloma cells of MM160 and U266B1, and (ii) presentation on HLA-B*07:02 and HLA-B*51:01. Peptides with predicted HLA binding affinity ≤ 700 nmol/L (NetMHC) were considered presented. D, L-363 cells that did not activate 58-16A2 without additional peptide loading were used as antigen-presenting cells. RPR (amino acid sequence: RPRPPVLSV) was used as a negative control peptide representative for all other 274 nonamer peptides that did not activate 58-16A2. E, Combined single-cell gene expression of CD38-enriched multiple myeloma bone marrow and peripheral blood cells from patient MM160. Each data point represents 1 of 4,430 single cells. Left: Multiple myeloma cells were identified by monoclonal immunoglobulin light chain CDR3 sequences. Right: AP5M1 expression was generally low and not restricted to multiple myeloma cells. A, B, and D indicate mean ± standard deviation. Data are representative of 3 experiments unless otherwise stated.