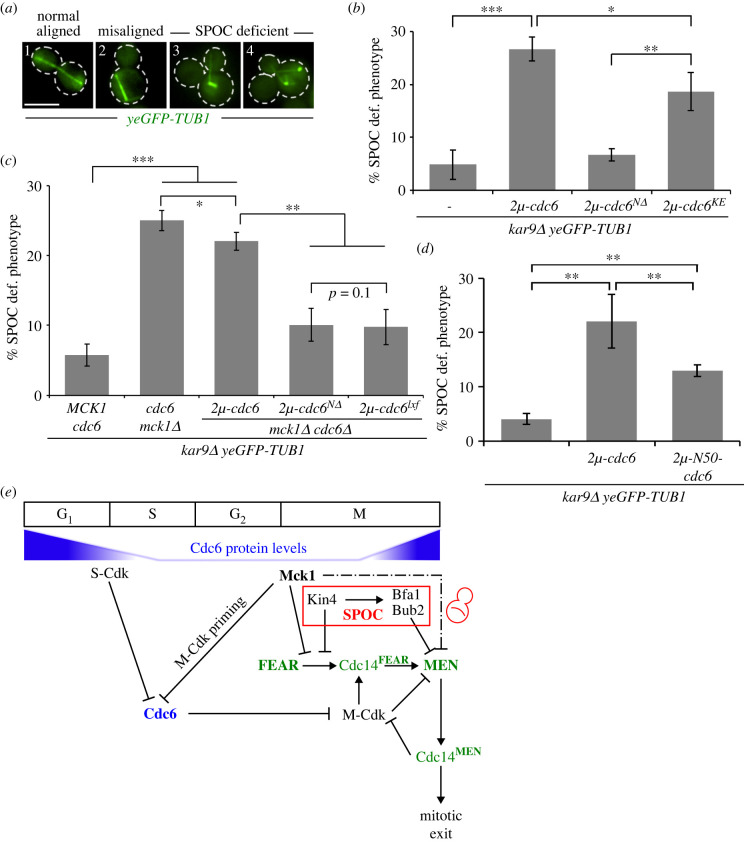
Figure 6.
The N-terminal domain of Cdc6 inhibits mitotic exit in cells with misaligned spindle. (a) Representative images of cells that were fixed and analysed for yeGFP-tubulin. Scale bar, 3 μm. (b–d) Analysis of SPOC in kar9Δ yeGFP-TUB1 strains carrying high-copy 2µ-plasmids expressing CDC6 full length, truncated forms or mutants as indicated. Graphs show the average ± s.d. of the percentage of cells with SPOC deficient phenotypes scored from three independent experiments. N = 100 anaphase cells per strain and experiment. Asterisk indicates a significant difference based on the two-tailed Student's t-test, p < 0.05 (*), p < 0.001 (**) and p < 0.0001 (***). (e) Model depicting the role of Mck1 and Cdc6 in SPOC and mitotic exit regulation. Cdc6 is degraded at the G1/S phase by S-Cdk and during mitosis by the action of Mck1 with the help of M-Cdk (priming kinase for Mck1) [57,58,61]. Cdc6 starts re-accumulating at the end of mitosis, where it helps to inhibit M-Cdk activity to promote mitotic exit [58]. Cells lacking MCK1 or bearing high levels of Cdc6 throughout mitosis are SPOC deficient. Our data show that Mck1 and Kin4 work in parallel to prevent FEAR-dependent MEN activation (see text for details).