Abstract
In addition to showing antidiabetic effects, sodium–glucose cotransporter 2 (SGLT2) inhibitors also reduce cardiovascular events in patients with type 2 diabetes mellitus. In major trials of cardiovascular outcomes, SGLT2 inhibitors have been shown to improve cardiovascular and renal outcomes, including reduced rehospitalization in patients with heart failure, regardless of the presence of diabetes. A recent report showed that the benefits of SGLT2 inhibitors in terms of cardiovascular deaths/admissions caused by heart failure and reduced ejection fraction were greater in Asians than in Whites. In this review, the first part demonstrates the results of recent clinical trials and their clinical implications and outlines current trials and upcoming research areas. The second part provides a general overview of the current understanding of the mechanisms of the cardiovascular benefits of SGLT2 inhibitors.
Key Words: diabetes, heart failure, sodium–glucose cotransporter 2 inhibitor
Abbreviations and Acronyms: ATP, adenosine triphosphate; DPP-4, dipeptidyl peptidase-4; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; SGLT2, sodium–glucose cotransporter 2; T2DM, type 2 diabetes mellitus
Central Illustration
Highlights
-
•
Type 2 diabetes mellitus and heart failure are closely related.
-
•
SGLT2 inhibitors can reduce the incidence of cardiovascular events and heart failure.
-
•
SGLT2 inhibitors can improve hemodynamics, myocardial energy supply, and sympathetic and parasympathetic nerve activities.
The incidence of heart failure (HF) is increasing worldwide.1 Type 2 diabetes mellitus (T2DM) and HF are closely related to each other,2, 3, 4 and approximately 45% of people with HF have diabetes.5 The risk of HF is correlated with hemoglobin A1c levels.6,7 HF is the most common cause of hospitalization caused by cardiovascular events in patients with diabetes.8 T2DM and HF have independent prognoses.9, 10, 11 In Asia, an aging population and a significant increase in cardiovascular risk factors have affected the HF burden.12 Asian patients with HF and a reduced ejection fraction (HFrEF) show clinical features different from other patients.13,14 The ASIAN-HF (Asian Sudden Cardiac Death in Heart Failure) registry was established to address the lack of knowledge of the burden associated with chronic HF among Asian patients.15 In this prospective observational cohort, the comorbidities of diabetes had the largest influence on the combined outcome of death and hospital admission caused by HF.16 Based on evaluations using the ASIAN-HF registry, Asian patients with HF had a lower mean body mass index and were less likely to be in New York Heart Association functional class III or take angiotensin-converting enzyme inhibitors.17
The high prevalence of T2DM in Asia has the potential to cause an outbreak of cardiovascular diseases and HF, so prevention of cardiovascular complications is essential in managing patients with T2DM.18,19 The current prevalence rates of T2DM and cardiovascular diseases and estimates of future risk in Asia are alarming and require effective action to prevent disease development and ensure effective management of T2DM and cardiovascular diseases. In recent large randomized placebo-controlled trials, sodium–glucose cotransporter 2 (SGLT2) inhibitors were shown to decrease cardiovascular events, specifically secondary prevention and hospital admission for HF.20, 21, 22, 23, 24, 25, 26 Recent reviews have focused on the clinical benefits and mechanisms of the cardiorenal effects of SGLT2 inhibitors.27, 28, 29 In this review, the first part demonstrates the results of recent clinical trials and their clinical implications and outlines current trials and upcoming research areas. The second part provides a general overview of the current understanding of the mechanisms of the cardiovascular benefits of SGLT2 inhibitors. As a unique point, we show on diabetes and HF as well as SGLT2 inhibitors in the Asian population.
SGLT2 Inhibitors and HFrEF
In 4 cardiovascular outcome trials (ie, EMPA-REG OUTCOME [Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients], CANVAS [Canagliflozin Cardiovascular Assessment Study], DECLARE-TIMI 58 [Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events-Thrombolysis In Myocardial Infarction 58], and CREDENCE [Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy])20, 21, 22, 23 (Table 1) and 3 HF-specific trials (ie, DAPA-HF [Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure], EMPEROR-Reduced [Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction], and SOLOIST-WHF [Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure)25,26,30 (Table 2), SGLT2 inhibitors significantly decreased the risk of all-cause mortality, cardiovascular mortality, and hospitalization caused by HF in both the presence and absence of T2DM. A trend toward reduced HF hospitalization-related outcomes was observed in the subgroup of patients with HF with preserved ejection fraction (HFpEF), but this conclusion was not definitive and should be considered exploratory. In comparison with placebo, SGLT2 inhibitors have not been shown to increase the risk of severe adverse events or discontinuation of treatment after adverse events. The 3 HF-specific trials also showed significant reductions in the composite outcome of initial hospitalization caused by HF or cardiovascular deaths. Although the DAPA-HF trial showed a significant reduction in mortality caused by cardiovascular and other causes, EMPEROR-Reduced and SOLOIST-WHF did not. A meta-analysis of these 3 trials showed a significant decrease in cardiovascular mortality from all causes.31 The results of a post hoc analysis of the results of cardiovascular trials further supported the benefits of reduced mortality in patients with HF.31 Subsequent analyses in the DAPA-HF trial reported that the benefits of dapagliflozin were significant in both patients with and without diabetes.32 Several other ongoing trials have focused on the role of SGLT2 inhibitors in patients with HFpEF, with or without T2DM. The mechanism underlying the benefits of SGLT2 inhibitors for the prognosis of patients with HFrEF is unclear, but some studies have suggested that they include beneficial effects on myocardial metabolism, fibrosis, inflammation, and vascular function.33, 34, 35
Table 1.
4 Cardiovascular Outcome Trials Involving SGLT2 Inhibitors
| EMPA-REG OUTCOME20 | CANVAS Program21 | DECLARE-TIMI 5822 | CREDENCE23 | |
|---|---|---|---|---|
| SGLT2 inhibitor | Empagliflozin | Canagliflozin | Dapagliflozin | Canagliflozin |
| Median duration of follow-up, y | 3.1 | 2.4 | 4.2 | 2.6 |
| All | 7,020 | 10,142 | 17,160 | 4,401 |
| Asian | 1,517 (21.6) | 1,248 (12.7) | 2,303 (13.4) | 877 (19.9) |
| MACE | HR: 0.86 (95% CI: 0.74-0.99); P = 0.04 | HR: 0.86 (95% CI: 0.75-0.97); P = 0.02 | HR: 0.93 (95% CI: 0.84-1.03); P = 0.17 | HR: 0.80 (95% CI: 0.67-0.95); P = 0.01 |
| CV death | HR: 0.62 (95% CI: 0.49-0.77); P < 0.001 | HR: 0.87 (95% CI: 0.75-0.97) | HR: 0.83 (95% CI: 0.73-0.95); P = 0.005 | HR: 0.78 (95% CI: 0.61-1.00); P = 0.05 |
Values are n or n (%) unless otherwise indicated.
CANVAS = Canagliflozin Cardiovascular Assessment Study; CREDENCE = Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy; CV death = cardiovascular death; DECLARE-TIMI 58 = Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events-Thrombolysis In Myocardial Infarction 58; EMPA-REG OUTCOME = Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; MACE = major adverse cardiovascular events including a composite of death from cardiovascular causes; SGLT2 = sodium–glucose cotransporter 2.
Table 2.
3 HF-Specific Trials Involving SGLT2 Inhibitors
| DAPA HF25 | EMPEROR-Reduced26 | SOLOIST-WHF30 | |
|---|---|---|---|
| SGLT2 inhibitor | Dapagliflozin | Empagliflozin | Sotagliflozin |
| Median duration of follow-up, m | 18.2 | 16 | 9 |
| All | 4,744 | 3,730 | 1,222 |
| Asian | 1,076 (22.7) | 493 (13.2) | 15 (1.2) |
| Primary outcomea | HR: 0.74 (95% CI: 0.65-0.85); P < 0.001 | HR: 0.75 (95% CI: 0.65-0.86); P < 0.001 | HR: 0.67 (95% CI: 0.52-0.85); P < 0.001 |
| CV death | HR: 0.82 (95% CI: 0.69-0.98) | HR: 0.92 (95% CI: 0.75-1.12) | HR: 0.84 (95% CI: 0.58-1.22); P = 0.36 |
Values are n or n (%) unless otherwise indicated.
DAPA-HF = Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure; EMPEROR-Reduced = Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction; SOLOIST – WHF = Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure; other abbreviations as in Table 1.
Composite of worsening heart failure (hospitalization or an urgent visit resulting in intravenous therapy for heart failure) or cardiovascular death.
SGLT2 inhibitors and HFpEF
In contrast to the findings for HFrEF, the effects of SGLT2 inhibitors on patients with HFpEF are still limited. Data from a pooled analysis including the DECLARE-TIMI 58 trial, the SOLOIST-WHF, and SCORED (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) trials showed borderline significant reduction in the composite outcome of HF hospitalization or cardiovascular death.31 EMPEROR-PRESERVED (Enpagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction), in which 11% of the patients were Asians, enrolled 5,988 patients with HFpEF with and without T2DM. The primary outcome was hospitalization for HF or cardiovascular mortality. The results indicated that empagliflozin reduced HF and cardiovascular mortality.36 The DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure; NCT01297257) trial is ongoing.
SGLT2 inhibitors in asia
In a pooled analysis, empagliflozin was shown to be well-tolerated by East Asian patients with T2DM considering an exposure of more than 2,100 patient-years, consistent with the results for the overall population tested.37 Empagliflozin has also been shown to reduce the risk of cardiovascular outcomes and mortality not only in the overall study population but also in Asian patients with T2DM and a history of cardiovascular disease.38 In a large, international study involving patients with T2DM from the Asia Pacific, the Middle East, and North America, the use of SGLT2 inhibitors decreased cardiovascular events in a broad evaluation of patient outcomes and characteristics.39 Empagliflozin treatment is also associated with a lower risk of HF, all-cause mortality, and end-stage renal disease in comparison with dipeptidyl peptidase-4 (DPP-4) inhibitors in routine clinical practice in Japan, South Korea, and Taiwan.40 One study suggested that the use of SGLT2 inhibitors afforded cardiovascular disease protection and could be used safely in older adults with T2DM.41 In the meta-analysis of Asians, the effects of empagliflozin and dapagliflozin on hospitalization caused by HF were shown to be similar in 2 independent trials, suggesting that these drugs improve renal outcomes and reduce all-cause and cardiovascular mortality in patients with HFrEF.42 Similarly, in Asia, SGLT2 inhibitor treatment is an evidence-based therapeutic regimen for primary prevention of hospitalizations caused by HF and secondary prevention of cardiovascular events in patients with T2DM.18 Thus, SGLT2 inhibitors should be considered for additional dosing early in patients with multiple risk factors or pre-existing cardiovascular disease. A recent meta-analysis has shown that the benefits of SGLT2 inhibitors in Asians were greater than in Whites in terms of cardiovascular deaths/admissions caused by HF in patients with HFrEF.43
Comparison with other antidiabetic drugs
Dapagliflozin has been reported to reduce the risk of HF and direct medical costs in comparison with DPP-4 initiators in Asian countries.44 Additionally, SGLT2 inhibitors have been shown to reduce the risk of cardiovascular events in comparison with initiators of other hypoglycemic agents and DPP-4 initiators in Japanese real-world practice.45 Although both SGLT2 inhibitors and glucagon-like peptide-1 receptor agonists were reported to reduce all-cause mortality, cardiovascular mortality, nonfatal myocardial infarction, and renal failure, SGLT2 inhibitors reduced mortality and hospitalization caused by HF more frequently than glucagon-like peptide-1 receptor agonists.46
Mechanism of the HF improvements associated with SGLT2 inhibitors
The beneficial effects of SGLT2 inhibitors on hemodynamics, myocardial energy supply, and sympathetic and parasympathetic nerve activities are illustrated in Figure 1.47,48
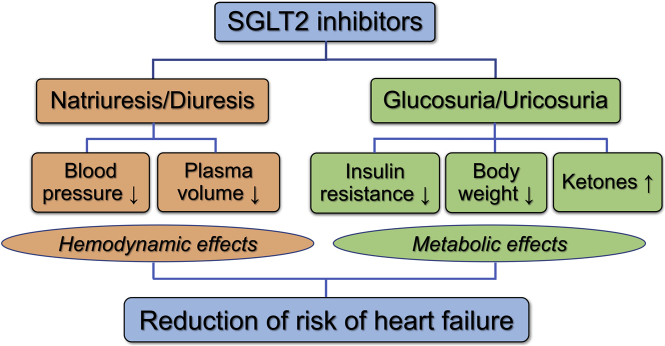
Figure 1.
Main Mechanisms of Action of SGLT2 Inhibitors
Their metabolic and hemodynamic effects improve myocardial function and reduce the risk of heart failure (details are provided in the text).47 SGLT2 = sodium–glucose cotransporter 2.
Hemodynamic effect
The combination of the natriuretic and osmotic effects of SGLT2 inhibitors reduces intracellular and extracellular volumes to the same extent.49,50 The sustained reduction in intravascular volume and blood pressure reduces preload and postoperative load of the heart,51, 52, 53 respectively, alleviating the cardiac workload, and improving left ventricular function.54 SGLT2 inhibitors reduce reflex sympathetic hyperactivity and affect other neurohormonal pathways that affect the heart by altering intravascular volume and blood pressure hemodynamics, but do not increase the heart rate.55,56 Several clinical studies have reported significant body weight reductions in patients treated with SGLT2 inhibitors.57,58
Myocardial energy supply effect
SGLT2 inhibitors increase the circulatory rates of ketone bodies.59 Ketones are freely absorbed by myocardial cells and can be a more effective source of adenosine triphosphate (ATP) than fatty acids for the failing heart.60 Additionally, the rate of use of ketones is reduced during myocardial ischemia.61 An experimental study reported that the increased use of fatty acids, ketone bodies, and branched-chain amino acids with empagliflozin inhibited the reduction of ATP and increased myocardial ATP levels.62 The mechanism underlying these effects involves the activation of the signal transducer and transcription activator 3, which, therefore, has antioxidant and anti-inflammatory activities.63 Several experimental and human studies have shown the beneficial effects of SGLT2 inhibition on cardiac remodeling.64, 65, 66, 67, 68, 69, 70 In a basic experimental design of acute myocardial infarction, SGLT2 inhibitors preserved heart function and reduced the infarct size.63 Furthermore, SGLT2 inhibitors have been reported to increase erythropoietin, which has cardioprotective effects, and hemoglobin, which enhances the oxygen supply to the myocardium.71,72 Additionally, SGLT2 inhibitors have been hypothesized to directly inhibit sodium–hydrogen (Na+/H+) exchange in the myocardium, resulting in an increase in mitochondrial calcium levels, improvement in mitochondrial function, reduction of oxidative stress, and reduction of arrhythmias.33 All of these mechanisms strongly suggest that SGLT2 inhibitors have cardioprotective effects.
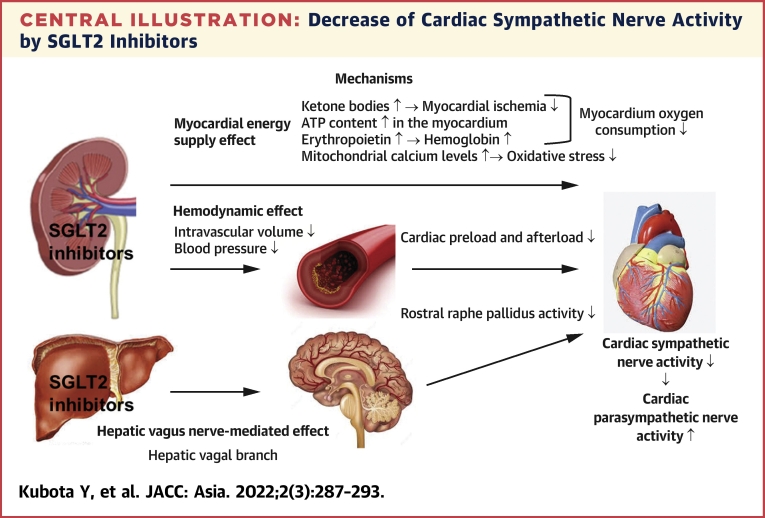
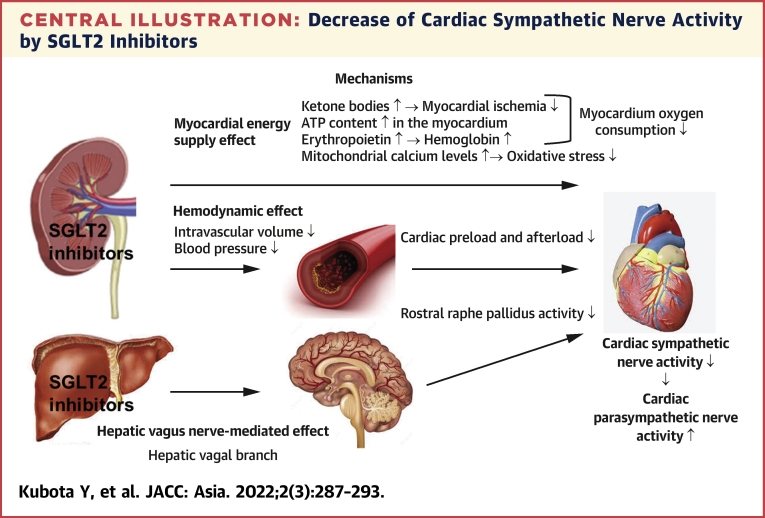
Effect on sympathetic and parasympathetic nerve activities
In our EMBODY trial, a prospective randomized placebo-control trial in patients with acute myocardial infarction associated with T2DM, the cardiac sympathetic nerve activity was significantly decreased, and the parasympathetic nerve activity was significantly increased in only the empagliflozin group.73 The Central Illustration shows our proposed mechanism for the decrease of cardiac sympathetic nerve activity by SGLT2 inhibitors. There are considered to be 3 mechanisms to decrease the cardiac sympathetic nerve activity by SGLT2 inhibitors, involving a hemodynamic effect, a metabolic (myocardial energy supply) effect, and a hepatic vagus nerve-mediated effect. The decreases of both myocardium oxygen consumption (myocardial energy supply effect) and cardiac preload and afterload (hemodynamic effect)74 are easily expected to decrease the cardiac sympathetic nerve activity. The vagus nerve in the liver controls neuron activation in the rostral raphe pallidus, which promotes sympathetic activity in the heart and increases the heart rate.75 Administration of SGLT2 inhibitors can reduce the activity of the cardiac sympathetic nerve by reducing the activity of the cord and controlling the heart rate.76 The precise mechanism for the increase of cardiac parasympathetic nerve activity with SGLT2 inhibitors demonstrated by our EMBODY trial is unclear, which may be a secondary effect caused by the decrease of cardiac sympathetic nerve activity. In any case, both the decease of sympathetic nerve activity and the increase of parasympathetic nerve activity in the heart with SGLT2 inhibitors indicate that SGLT2 inhibitors have preventive effects on cardiac arrhythmias as well as exacerbation of HF.
Central Illustration.
Decrease of Cardiac Sympathetic Nerve Activity by SGLT2 Inhibitors
There are considered to be 3 mechanisms to decrease the cardiac sympathetic nerve activity by SGLT2 inhibitors, involving a hemodynamic effect, a metabolic (myocardial energy supply) effect, and a hepatic vagus nerve-mediated effect (details are provided in the text). ATP = adenosine triphosphate; SGLT2 = sodium–glucose cotransporter 2.
Safety of SGLT2 Inhibitors
SGLT2 inhibitors may increase the risk of fungal genital infections, urinary tract infections, and euglycemic diabetic ketoacidosis.77,78 In the CANVAS trial, an increased risk of bone fractures and lower limb amputations was reported only with canagliflozin.21 Dosage adjustments are not required in older patients; the risk of adverse events related to volume depletion, renal failure, or urinary tract infection is higher in patients ≥65 years of age.78 The risk of euglycemic diabetic ketoacidosis is higher in lean patients, those with decreased β-cell reserves, and those on a ketogenic diet.78
Conclusions
SGLT2 inhibitors can be used for the secondary prevention of cardiovascular outcomes in patients with T2DM and a history of cardiovascular disease in consideration of their beneficial cardiovascular and metabolic effects. SGLT2 inhibitors can also be used for primary and secondary prevention of HF-related hospitalization in patients with T2DM and multiple risk factors.
Funding Support and Author Disclosures
Dr Shimizu has received honorariums and/or scholarship funds from Boehringer Ingelheim Co, Ltd, Daiichi Sankyo Co, Ltd, Ono Pharmaceutical Co, Ltd, Bayer Co, Ltd, Pfizer Co, Ltd, and Bristol-Myers Squibb Co, Ltd. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Sanjiv J. Shah, MD, served as Guest Associate Editor for this paper. Yibin Wang, PhD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 2.van Melle J.P., Bot M., de Jonge P., de Boer R.A., van Veldhuisen D.J., Whooley M.A. Diabetes, glycemic control, and new-onset heart failure in patients with stable coronary artery disease: data from the Heart and Soul Study. Diabetes Care. 2010;33:2084–2089. doi: 10.2337/dc10-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seferović P.M., Petrie M.C., Filippatos G.S., et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853–872. doi: 10.1002/ejhf.1170. [DOI] [PubMed] [Google Scholar]
- 4.Dunlay S.M., Givertz M.M., Aguilar D., et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America. Circulation. 2019;140:e294–e324. doi: 10.1161/CIR.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 5.Echouffo-Tcheugui J.B., Xu H., DeVore A.D., et al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from Get With The Guidelines-Heart Failure registry. Am Heart J. 2016;182:9–20. doi: 10.1016/j.ahj.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Pazin-Filho A., Kottgen A., Bertoni A.G., et al. HbA 1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2008;51:2197–2204. doi: 10.1007/s00125-008-1164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert R.E., Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet. 2015;385:2107–2117. doi: 10.1016/S0140-6736(14)61402-1. [DOI] [PubMed] [Google Scholar]
- 8.Burrows N.R., Li Y., Gregg E.W., Geiss L.S. Declining rates of hospitalization for selected cardiovascular disease conditions among adults aged ≥35 years with diagnosed diabetes, U.S., 1998-2014. Diabetes Care. 2018;41:293–302. doi: 10.2337/dc17-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan S.S., Butler J., Gheorghiade M. Management of comorbid diabetes mellitus and worsening heart failure. JAMA. 2014;311:2379–2380. doi: 10.1001/jama.2014.4115. [DOI] [PubMed] [Google Scholar]
- 10.Swoboda P.P., McDiarmid A.K., Erhayiem B., et al. Diabetes mellitus, microalbuminuria, and subclinical cardiac disease: identification and monitoring of individuals at risk of heart failure. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association Cardiovascular disease and risk management: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S103–S123. doi: 10.2337/dc19-S010. [DOI] [PubMed] [Google Scholar]
- 12.Shimokawa H., Miura M., Nochioka K., Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17:884–892. doi: 10.1002/ejhf.319. [DOI] [PubMed] [Google Scholar]
- 13.Atherton J.J., Hayward C.S., Wan Ahmad W.A., et al. Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International-Asia Pacific) J Card Fail. 2012;18:82–88. doi: 10.1016/j.cardfail.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Mentz R.J., Roessig L., Greenberg B.H., et al. Heart failure clinical trials in East and Southeast Asia: understanding the importance and defining the next steps. J Am Coll Cardiol HF. 2016;4:419–427. doi: 10.1016/j.jchf.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam C.S., Anand I., Zhang S., et al. Asian Sudden Cardiac Death in Heart Failure (ASIAN-HF) registry. Eur J Heart Fail. 2013;15:928–936. doi: 10.1093/eurjhf/hft045. [DOI] [PubMed] [Google Scholar]
- 16.Yap J., Tay W.T., Teng T.K., et al. Association of diabetes mellitus on cardiac remodeling, quality of life, and clinical outcomes in heart failure with reduced and preserved ejection fraction. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam C.S., Teng T.K., Tay W.T., et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. 2016;37:3141–3153. doi: 10.1093/eurheartj/ehw331. [DOI] [PubMed] [Google Scholar]
- 18.Deerochanawong C., Chan S.P., Matawaran B.J., et al. Use of sodium-glucose co-transporter-2 inhibitors in patients with type 2 diabetes mellitus and multiple cardiovascular risk factors: an Asian perspective and expert recommendations. Diabetes Obes Metab. 2019;21:2354–2367. doi: 10.1111/dom.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rydén L., Grant P.J., Anker S.D., et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 20.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 21.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 22.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 23.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 24.Zelniker T.A., Wiviott S.D., Raz I., et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 25.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 26.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 27.Zelniker T.A., Braunwald E. Clinical benefit of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:435–447. doi: 10.1016/j.jacc.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 28.Lopaschuk G.D., Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. J Am Coll Cardiol Basic Trans Science. 2020;5:632–644. doi: 10.1016/j.jacbts.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang C.E., Wang K.L., Cheng H.M., Sung S.H., Chao T.F. Second revolution in cardiovascular prevention. J Chin Med Assoc. 2020;83:327–336. doi: 10.1097/JCMA.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 30.Bhatt D.L., Szarek M., Steg P.G., et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 31.Butler J., Usman M.S., Khan M.S., et al. Efficacy and safety of SGLT2 inhibitors in heart failure: systematic review and meta-analysis. ESC Heart Fail. 2020;7:3298–3309. doi: 10.1002/ehf2.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrie M.C., Verma S., Docherty K.F., et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. doi: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Packer M., Anker S.D., Butler J., Filippatos G., Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2:1025–1029. doi: 10.1001/jamacardio.2017.2275. [DOI] [PubMed] [Google Scholar]
- 34.Verma S., McMurray J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 35.Lytvyn Y., Bjornstad P., Udell J.A., Lovshin J.A., Cherney D.Z.I. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 37.Yabe D., Yasui A., Ji L., et al. Safety and tolerability of empagliflozin in East Asian patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. J Diabetes Investig. 2019;10:418–428. doi: 10.1111/jdi.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaku K., Lee J., Mattheus M., Kaspers S., George J., Woerle H.J. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease-results from EMPA-REG OUTCOME®. Circ J. 2017;81:227–234. doi: 10.1253/circj.CJ-16-1148. [DOI] [PubMed] [Google Scholar]
- 39.Kosiborod M., Lam C.S.P., Kohsaka S., et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 Study. J Am Coll Cardiol. 2018;71:2628–2639. doi: 10.1016/j.jacc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Seino Y., Kim D.J., Yabe D., et al. Cardiovascular and renal effectiveness of empagliflozin in routine care in East Asia: results from the EMPRISE East Asia study. Endocrinol Diabetes Metab. 2021;4 doi: 10.1002/edm2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han S.J., Ha K.H., Lee N., Kim D.J. Effectiveness and safety of sodium-glucose co-transporter-2 inhibitors compared with dipeptidyl peptidase-4 inhibitors in older adults with type 2 diabetes: a nationwide population-based study. Diabetes Obes Metab. 2021;23:682–691. doi: 10.1111/dom.14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zannad F., Ferreira J.P., Pocock S.J., et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 43.Lee M.M.Y., Ghouri N., McGuire D.K., Rutter M.K., Sattar N. Meta-analyses of results from randomized outcome trials comparing cardiovascular effects of SGLT2is and GLP-1RAs in Asian versus white patients with and without type 2 diabetes. Diabetes Care. 2021;44:1236–1241. doi: 10.2337/dc20-3007. [DOI] [PubMed] [Google Scholar]
- 44.Seong J.M., Kim J.J., Kim H.J., Sohn H.S. Comparison of heart failure risk and medical costs between patients with type 2 diabetes mellitus treated with dapagliflozin and dipeptidyl peptidase-4 inhibitors: a nationwide population-based cohort study. Cardiovasc Diabetol. 2020;19:95. doi: 10.1186/s12933-020-01060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohsaka S., Takeda M., Bodegård J., et al. Sodium-glucose cotransporter 2 inhibitors compared with other glucose-lowering drugs in Japan: subanalyses of the CVD-REAL 2 Study. J Diabetes Investig. 2021;12:67–73. doi: 10.1111/jdi.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer S.C., Tendal B., Mustafa R.A., et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inzucchi S.E., Zinman B., Wanner C., et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheen A.J. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ Res. 2018;122:1439–1459. doi: 10.1161/CIRCRESAHA.117.311588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masuda T., Murakami T., Igarashi Y., et al. Dual impact of tolvaptan on intracellular and extracellular water in chronic kidney disease patients with fluid retention. Intern Med. 2016;55:2759–2764. doi: 10.2169/internalmedicine.55.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hallow K.M., Helmlinger G., Greasley P.J., McMurray J.J.V., Boulton D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487. doi: 10.1111/dom.13126. [DOI] [PubMed] [Google Scholar]
- 51.Mazidi M., Rezaie P., Gao H.K., Kengne A.P. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber M.A., Mansfield T.A., Cain V.A., Iqbal N., Parikh S., Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211–220. doi: 10.1016/S2213-8587(15)00417-9. [DOI] [PubMed] [Google Scholar]
- 53.Kario K., Okada K., Kato M., et al. 24-hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, placebo-controlled SACRA Study. Circulation. 2018;139(18):2089–2097. doi: 10.1161/CIRCULATIONAHA.118.037076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sattar N., McLaren J., Kristensen S.L., Preiss D., McMurray J.J. SGLT2 inhibition and cardiovascular events: why did EMPA-REG outcomes surprise and what were the likely mechanisms. Diabetologia. 2016;59:1333–1339. doi: 10.1007/s00125-016-3956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chilton R., Tikkanen I., Cannon C.P., et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–1193. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–476. doi: 10.1016/j.jjcc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Cai X., Yang W., Gao X., et al. The association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta-analysis. Obesity (Silver Spring) 2018;26:70–80. doi: 10.1002/oby.22066. [DOI] [PubMed] [Google Scholar]
- 58.Lee P.C., Ganguly S., Goh S.Y. Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obes Rev. 2018;19:1630–1641. doi: 10.1111/obr.12755. [DOI] [PubMed] [Google Scholar]
- 59.Ferrannini E., Baldi S., Frascerra S., et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 60.Ferrannini E., Mark M., Mayoux E. CV protection in the EMPA-REG OUTCOME Trial: a "thrifty substrate" hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 61.Arima Y., Izumiya Y., Ishida T., et al. Myocardial ischemia suppresses ketone body utilization. J Am Coll Cardiol. 2019;73:246–247. doi: 10.1016/j.jacc.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 62.Oshima H., Miki T., Kuno A., et al. Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats. J Pharmacol Exp Ther. 2019;368:524–534. doi: 10.1124/jpet.118.253666. [DOI] [PubMed] [Google Scholar]
- 63.Andreadou I., Efentakis P., Balafas E., et al. Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects. Front Physiol. 2017;8:1077. doi: 10.3389/fphys.2017.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lambers Heerspink H.J., de Zeeuw D., Wie L., Leslie B., List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byrne N.J., Parajuli N., Levasseur J.L., et al. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. J Am Coll Cardiol Basic Trans Science. 2017;2:347–354. doi: 10.1016/j.jacbts.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Connelly K.A., Zhang Y., Visram A., et al. Empagliflozin improves diastolic function in a nondiabetic rodent model of heart failure with preserved ejection fraction. J Am Coll Cardiol Basic Trans Science. 2019;4:27–37. doi: 10.1016/j.jacbts.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi L., Zhu D., Wang S., Jiang A., Li F. Dapagliflozin attenuates cardiac remodeling in mice model of cardiac pressure overload. Am J Hypertens. 2019;32:452–459. doi: 10.1093/ajh/hpz016. [DOI] [PubMed] [Google Scholar]
- 68.Verma S., Mazer C.D., Yan A.T., et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation. 2019;140:1693–1702. doi: 10.1161/CIRCULATIONAHA.119.042375. [DOI] [PubMed] [Google Scholar]
- 69.Verma S., Garg A., Yan A.T., et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME Trial. Diabetes Care. 2016;39:e212–e213. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]
- 70.Esterline R.L., Vaag A., Oscarsson J., Vora J. Mechanisms in endocrinology: SGLT2 inhibitors: clinical benefits by restoration of normal diurnal metabolism. Eur J Endocrinol. 2018;178:R113–R125. doi: 10.1530/EJE-17-0832. [DOI] [PubMed] [Google Scholar]
- 71.Sano M., Takei M., Shiraishi Y., Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8:844–847. doi: 10.14740/jocmr2760w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van der Meer P., Lipsic E. Erythropoietin: repair of the failing heart. J Am Coll Cardiol. 2006;48:185–186. doi: 10.1016/j.jacc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu W., Kubota Y., Hoshika Y., et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19:148. doi: 10.1186/s12933-020-01127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoshika Y., Kubota Y., Shimizu W., et al. Effect of empagliflozin versus placebo on body fluid balance in patients with acute myocardial infarction and type 2 diabetes mellitus: subgroup analysis of the EMBODY trial. J Cardiac Fail. 2021;28:56–64. doi: 10.1016/j.cardfail.2021.07.022. [DOI] [PubMed] [Google Scholar]
- 75.Cao W.H., Morrison S.F. Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res. 2003;980:1–10. doi: 10.1016/s0006-8993(03)02981-0. [DOI] [PubMed] [Google Scholar]
- 76.Chiba Y., Yamada T., Tsukita S., et al. Dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, acutely reduces energy expenditure in BAT via neural signals in mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inagaki N., Harashima S., Maruyama N., Kawaguchi Y., Goda M., Iijima H. Efficacy and safety of canagliflozin in combination with insulin: a double-blind, randomized, placebo-controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:89. doi: 10.1186/s12933-016-0407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Handelsman Y., Henry R.R., Bloomgarden Z.T., et al. American Association of Clinical Endocrinologists and American College Of Endocrinology Position Statement on the Association of SGLT-2 Inhibitors and Diabetic Ketoacidosis. Endocr Pract. 2016;22:753–762. doi: 10.4158/EP161292.PS. [DOI] [PubMed] [Google Scholar]