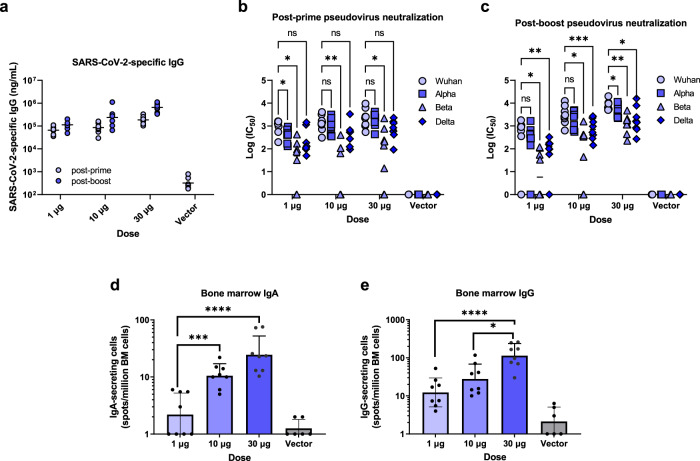
Fig. 4. Humoral immunogenicity profiles of optimized SARS-CoV-2 D614G-2P-3Q saRNA/NLC vaccine (AAHI-SC2) after prime or prime-boost immunization of C57BL/6 J mice.
a Serum SARS-CoV-2 spike protein-binding IgG. Horizontal lines show geometric mean. Serum SARS-CoV-2 neutralizing antibody titers post-prime (b) and post-boost (c). Horizontal lines show geometric mean. Data were log-transformed and evaluated by mixed effects analysis with multiple comparisons. Induction of bone marrow (BM)-resident IgA- (d) and IgG-secreting (e) cells by ELISpot. Results show geometric mean and geometric standard deviation. Analyzed with one-way ANOVA with multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. The vector control represents mice injected with 10 μg of NLC-complexed saRNA expressing the non-immunogenic secreted embryonic alkaline phosphatase (SEAP) gene. n = 6 mice for SEAP control group and n = 8 for dosing groups.