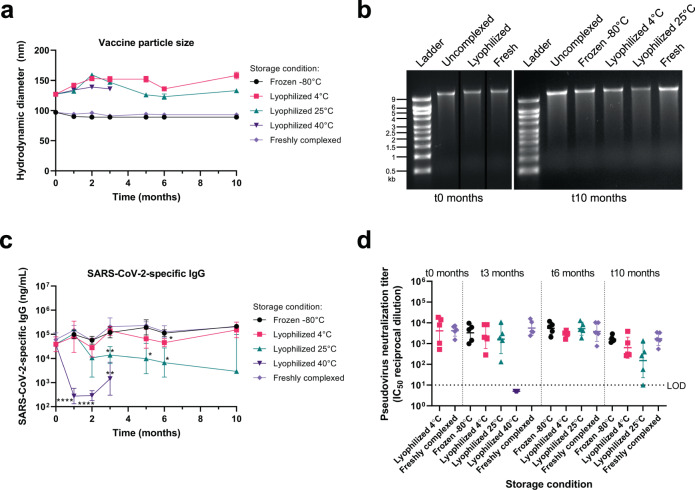
Fig. 6. Stability of the lyophilized SARS-CoV-2 saRNA/NLC vaccine (AAHI-SC2) after 10 months of storage at different temperatures.
a Mean hydrodynamic (Z-average) diameter of the vaccine complex with error bars indicating the standard deviation (SD) of n = 3 replicate measurements for each condition at each timepoint. b Integrity of vaccine RNA at 0 and 10 months of storage at the indicated temperatures. “Uncomplexed” refers to saRNA alone that had been stored at −80 °C for the indicated length of time. Lanes were derived from the same gel and re-arranged for the 0 months of storage image. See Supplementary Fig. 8 for original unprocessed gel images. c Serum SARS-CoV-2 spike protein-binding IgG induced in female C57BL/6J mice by the vaccine after storage under the indicated conditions for the indicated times. n = 5 mice per condition per timepoint. Antibody plots show geometric mean and geometric SD. Statistical analysis conducted on log-transformed data by Welch’s ANOVA test with Dunnett’s T3 multiple comparison test at each timepoint, comparing each stored vaccine preparation to freshly complexed vaccine. *p < 0.05, **p < 0.01, ****p < 0.0001. d Pseudovirus neutralization titers induced in female C57BL/6 J mice by the vaccine after storage under the indicated conditions for the indicated times. LOD = limit of detection. n = 5 mice per condition per timepoint. Results show geometric mean and geometric SD. Statistical analysis conducted on log-transformed data by Welch’s ANOVA test with Dunnett’s T3 multiple comparison test at each timepoint, comparing each stored vaccine preparation to freshly complexed vaccine.