Abstract
In recent years concern has mounted regarding the possibility of a re-emergence of smallpox through biowarfare or bioterrorism. There is also concern over the incidence of human monkeypox in endemic areas and the potential for monkeypox to be accidentally transported to non-endemic areas. In the event of re-emergence of smallpox or emergence of monkeypox, the accepted route of administration for live replicating smallpox vaccine is dermal scarification, which generates a virus-shedding lesion that persists for several days at the vaccination site. The lesion is a potential source of contact transmission of vaccine to individuals who may be contra-indicated for receipt of the live vaccine. In this study, we compare dermal scarification with intramuscular vaccination for replicating smallpox vaccine in a mouse lethal challenge model. Comparisons are made over multiple vaccine and challenge doses and data recorded for lethality, disease severity, and antibody responses. Qualitative and quantitative differences between the two routes are observed, and for the intramuscular route the febrile response is not suppressed after subsequent virulent vaccinia virus challenge. However both routes generate an immune response and protect from severe disease and death. Although dermal scarification is the preferred route of vaccination for the general population, intramuscular vaccination may be an option for people who are not contraindicated for the live vaccine, but who are close contacts of people who are contraindicated for the live vaccine, in an emergency situation.
Keywords: Vaccinia, Smallpox, Monkeypox, Intramuscular vaccination, Febrile response
1. Introduction
Variola virus (VARV, the causative agent of smallpox) and monkeypox virus (MPXV) are orthopoxvirinae. Orthopoxviruses are sufficiently related that infection with one can induce protection against the others, which facilitated use of vaccinia virus (VACV) as a live vaccine against both smallpox and monkeypox.
Traditional smallpox vaccine has side-effects associated with vaccine-virus replication (e.g. generalised vaccinia; progressive vaccinia; eczema vaccinatum; myopericarditis). The vaccine causes an open lesion which can result in accidental inoculation of sites such as eyes and genitalia, and transfer to contacts. There are known contra-indications which allow identification of some individuals at risk, but this cannot always prevent transfer to such people. There have been life-threatening adverse effects in both vaccinees and contacts in recent years [1], [2], [3].
Newer replicating vaccines produced in cell culture are expected to have the same side-effect profile [4], [5], [6]. The needs of people with contra-indications are likely to be met with a non-replicating vaccine (Modified Vaccinia Ankara; MVA) [7], [8], [9] or a highly attenuated replicating vaccine strain (Lc16M8) [10], [11], [12], [13], [14]. However, in the event of a smallpox emergency the majority of available vaccine will be the newer replicating vaccines based on the Lister and Dryvax traditional vaccines.
The traditional vaccine is administered by dermal scarification, causing an open skin lesion. Previous human studies have shown that intramuscular (i.m.) vaccination avoids open lesions but induces a qualitatively different immune response. Intramuscular vaccination would reduce the incidence of mild (autoinoculation) and severe (eczema vaccinatum and progressive vaccinia in contacts) side-effects. However it is not known if it would give suitable protection [15].
The MVA vaccine is administered by i.m. or subcutaneous injection, and protects animals from orthopoxvirus disease [16], [17], [18], [19], [20], [21], [22], [23]. Here we compare efficacy of the Lister vaccine by i.m. injection and scarification in a mouse lethal model. We have previously used this model to compare historical and replacement Lister vaccines, and the MVA strain [18], [24]. We show here that i.m. administration of replicating Lister vaccine is equivalent to scarification for efficacy in mice, and avoids open lesions. There are quantitative differences in both immune response and disease severity. While scarification remains the recommended route for administration of live smallpox vaccines, our data indicate that in an emergency, i.m. injection is worthy of consideration for people with no contraindications but who have close contacts who are contraindicated and at risk of vaccine transfer.
2. Methods
2.1. Study design
6–8 week old female Balb/c mice were purchased from Charles River, UK. All mice were identified by unique microchip, and habituated for one week before sorting into 36 random groups of 5 mice per cage. Vaccinations and challenges were administered as described below and in Table 1 , with vaccinations given on day 1, and challenges on day 28. Sham vaccinations used PBS. Surviving mice were humanely culled on day 42. Blood was drawn from the tail vein of subsets (n = 5 per subset) of mice at each vaccination dose on days 7, 14, 21 and 28. The same mice were used for each successive blood sample. The dose range for vaccination was selected to have a median of the human vaccination dose, not adjusted for weight. The challenge doses were selected to be as high as possible for the route of delivery.
Table 1.
Vaccination and challenge groups.
| Challenge (WR) dose (pfu/mouse) | 1 × 107 | 1 × 108 | 3.4 × 108 | 1 × 107 | 1 × 108 | 3.4 × 108 | Totals |
|---|---|---|---|---|---|---|---|
| Vaccine (Lister) dose (pfu/mouse) | Scarified (n) | Intramuscular (n) | |||||
| 1 × 103 | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| 1 × 104 | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| 1 × 105 | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| 1 × 106 | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| 1 × 107 | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| PBS | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| Grand total | 180 | ||||||
2.2. Viruses
VACV strains Lister and WR were purchased from the Swiss Serum Institute (SSI) and the ATCC respectively. Viruses were cultured in RK13 rabbit kidney cells in DMEM with 2% foetal bovine serum (FBS), 3 mM glutamine, and 100 units/ml penicillin and streptomycin. Virus was harvested by Dounce homogenisation of infected cells, and purified by ultra-centrifugation through a sucrose cushion. Virus was quantified by plaque titration on monolayers of RK13 cells, and stored at −80 °C.
2.3. Vaccinations
A single dose of Lister vaccine suspended in PBS was administered to mice as described [18]. For scarification, up to 1 × 107 pfu of vaccine, or sham (PBS) control was applied to a shaved flank in a 10 µl volume, and the area gently scarified with a 27 gauge needle using five strokes left-to-right and five strokes top-to-bottom. Intramuscular administration was by injection of up to 1 × 107 pfu of vaccine or sham (PBS) control into the unshaved flank in a 20 µl volume. Flanks were shaved 24 h prior to vaccination. Mice were housed with access to food and water ad libitum, and studies were in accordance with the UK Scientific Procedures (Animals) Act 1986, and UK Codes of Practice for the Housing and Care of Animals used in Scientific Procedures 1989.
2.4. ELISA
ELISAs were performed as previously described [25]. Up to 50 µl of blood was drawn from the tail vein and centrifuged at 10,000g for 10 min at room temperature, and serum collected. ELISA antigen was VACV Lister, coated onto ELISA plates at 1 × 106 plaque forming units (pfu)/well. Excess binding capacity was adsorbed with 2% milk powder, after which sera were applied at a 1/10 dilution in PBS in 100 µl volumes, with two wells per sample. Specific binding was detected with biotinylated goat anti-mouse IgG antibody, visualized with streptavidin-conjugated horse radish peroxidase and ABTS (Sigma). Optical density was measured with a 405 nm filter.
2.5. Mouse challenges
For challenge experiments, 1 × 107, 1 × 108, or 3.4 × 108 pfu of VACV strain WR suspended in PBS was administered in 10 μl to a single nares without anaesthesia. Challenges were administered 28 days after vaccination. Daily weights, temperatures and clinical signs were recorded. Temperatures were measured with a digital probe held in the forelimb axilla. All temperature measurements were performed by the same operator for consistency. Humane endpoints were either 30% body weight loss or acute clinical signs. Experiments ended 14 days after challenge.
2.6. Clinical scoring
Animals were scored twice daily after challenge, for head oedema; head shaking; eye problems; respiratory problems; rales; hunched posture; and ruffled fur. Each mouse was given a score of 1 for each condition that occurred, and if it achieved a score of 5 or more at any single check, weight loss of ≥30%, or was unable to open both eyes, it was humanely culled.
2.7. Statistical analysis
Data from individual mice was subjected to analysis of variance (ANOVA) or 2-way ANOVA. Daily weight was calculated as a percentage of initial weight. Minimum daily weight for each mouse post-vaccination and post-challenge was used for comparisons, as were the minimum and maximum temperatures.
3. Results
The Lister vaccine was administered to mice by scarification or i.m. routes, and the mice subsequently challenged with a range of doses of VACV strain WR, rising to the maximum that could be administered by the intranasal (i.n.) route. In the interval between vaccination and challenge, temperature differences were observed between scarification and i.m. groups (Fig.1 A and B). There was no significant difference between the routes for minimum temperature of mice over the 28 day post vaccination period (P = 0.36, 2-way ANOVA). However, there was a significant difference for maximum temperature, with the temperature for i.m. vaccinated mice depressed by an average of 0.48 °C relative to scarified mice (P = 6.45 × 10−11, 2-way ANOVA). There was no difference between the maximum temperature for control groups sham inoculated with PBS by the two routes (P = 0.11, ANOVA), or between individual scarification doses and the sham scarified group ((P ≥ 0.12, ANOVA). However, for the i.m. route, the maximum temperature during the post vaccination period was depressed in all groups relative to the sham vaccinated controls (P ≤ 0.0006, ANOVA, average depression of 0.66 °C).
Fig. 1.
Temperature (A, B) and weight (C, D) profiles of animals vaccinated by the scarification (A, C) or i.m. (B, D) route. Six- to eight-week-old female Balb/c mice were vaccinated with VACV strain Lister at doses of: 1 × 103 ( ); 1 × 104 (
); 1 × 104 ( ); 1 × 105 (
); 1 × 105 ( ); 1 × 106 (
); 1 × 106 ( ); or 1 × 107 (
); or 1 × 107 ( ) pfu/animal as described in Materials and Methods, or sham vaccinated with PBS(
) pfu/animal as described in Materials and Methods, or sham vaccinated with PBS( ). Animals were monitored daily for temperature and weight. Percentage weight was calculated as the percentage of initial weight for each animal. Data is presented as the daily mean temperature and percentage weight for each group. Group size = 10. Data from individual mice was subjected to 2-way ANOVA to compare the two vaccination routes, and a series of ANOVAs to compare each vaccination dose with the cognate mock vaccinated group. A further 5 mice for each group were not monitored for post-vaccination weight or temperature. These additional mice were required to provide enough animals for subsequent challenge procedures. The animals not monitored for post-vaccination temperature and weight were all assigned to the 3.4 × 108 pfu challenge groups in subsequent procedures.
). Animals were monitored daily for temperature and weight. Percentage weight was calculated as the percentage of initial weight for each animal. Data is presented as the daily mean temperature and percentage weight for each group. Group size = 10. Data from individual mice was subjected to 2-way ANOVA to compare the two vaccination routes, and a series of ANOVAs to compare each vaccination dose with the cognate mock vaccinated group. A further 5 mice for each group were not monitored for post-vaccination weight or temperature. These additional mice were required to provide enough animals for subsequent challenge procedures. The animals not monitored for post-vaccination temperature and weight were all assigned to the 3.4 × 108 pfu challenge groups in subsequent procedures.
With respect to post-vaccination weight there were also differences between scarification and i.m. routes (Fig.1C and D). There was no difference between the routes for the minimum weight post vaccination (P = 0.2, 2-way ANOVA). However, i.m. vaccinated mice gained significantly more weight than scarified mice over this period (P = 7.12 × 10−5, 2-way ANOVA, average percentage difference 2.8%). There was no difference between the maximum percentage weight gain for control groups sham inoculated with PBS by the two routes (P = 0.2, ANOVA), or between individual scarification doses and sham scarified controls, although the 103 pfu scarification group fell between the 95% and 99% confidence limits (P = 0.022; other groups P ≥ 0.24, ANOVA). However, for the i.m. route, the maximum percentage weight gain was elevated in several of the groups relative to the sham vaccinated control group (P ranges from 0.0084 to 0.74).
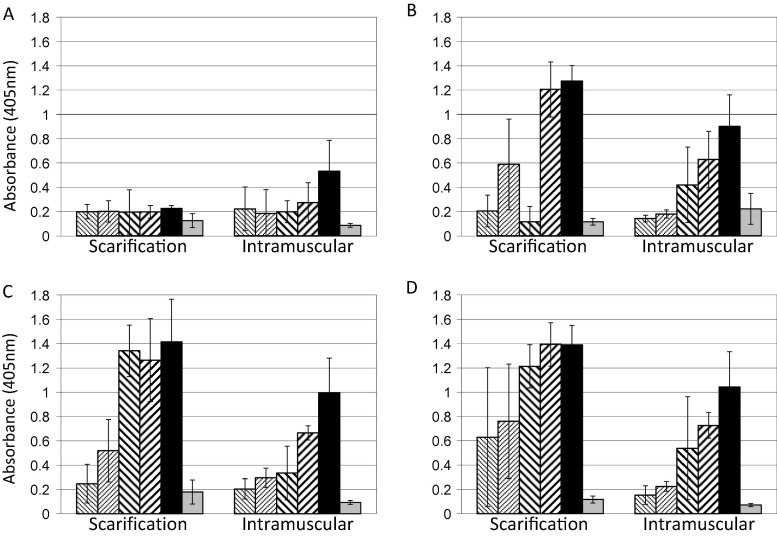
Subsets of animals were monitored weekly for serum antibody by ELISA. Significant differences were observed in the rate at which the anti-VACV antibody response developed, with the i.m. route stimulating a more rapid development of anti-VACV IgG antibody in serum than scarification at the highest vaccine dose, apparent at day 7 (P = 1.29 × 10−7, 2-way ANOVA); although the ultimate level of response was stronger in scarified animals, and observed at lower vaccine doses (Fig. 2 ). All scarified animals developed a lesion at the vaccination site. However no animals vaccinated by the i.m. route developed an observable lesion, at any vaccine dose (not shown).
Fig. 2.
ELISA of sera from vaccinated animals. Subsets (n = 5) of the animals described in Fig. 1 receiving vaccine at doses of 1 × 103 ( ); 1 × 104 (
); 1 × 104 ( ); 1 × 105 (
); 1 × 105 ( ); 1 × 106 (
); 1 × 106 ( ); or 1 × 107 (
); or 1 × 107 ( ) pfu/animal, or sham vaccinated with PBS(
) pfu/animal, or sham vaccinated with PBS( ), were selected for blood collection for serology. Animals were sequentially bled at 7 (A), 14 (B), 21 (C), and 28 (D) days post vaccination. Sera were prepared and used as described in Material and Methods. Data is shown as means and standard deviations of the results from the 5 mice in each subset.
), were selected for blood collection for serology. Animals were sequentially bled at 7 (A), 14 (B), 21 (C), and 28 (D) days post vaccination. Sera were prepared and used as described in Material and Methods. Data is shown as means and standard deviations of the results from the 5 mice in each subset.
At 28 days post-vaccination, mice were challenged with VACV strain WR as described in Methods, with increasing doses up to the maximum that could be administered without anaesthesia. Lethality was dependent on challenge dose in sham-vaccinated controls (Fig. 3 ), while disease severity was dependent on both challenge and vaccine dose in vaccinated animals.
Fig. 3.
Lethality of i.n. challenge in mock vaccinated controls. Control animals described in Fig. 1 were mock vaccinated with PBS by the scarification (filled symbols) or i.m. (empty symbols) routes and challenged i.n. 28 days later with VACV strain WR at 1 × 107 ( ,
,  ), 1 × 108 (
), 1 × 108 ( ,
,  ), or 3.4 × 108 (
), or 3.4 × 108 ( ,
,  ) pfu/animal. Surviving animals were recorded daily until 14 days post-challenge, when remaining animals were culled. Group size = 5.
) pfu/animal. Surviving animals were recorded daily until 14 days post-challenge, when remaining animals were culled. Group size = 5.
Differences between the vaccination routes were observed in temperature measurements (Fig. 4 ). For scarification, there was no difference between maximum temperatures pre- and post-challenge for the 107 and 108 pfu challenge doses, over all vaccine doses (P = 0.08 and 0.3 respectively, 2-way ANOVA). A significant difference was observed at the highest challenge dose, where the post-challenge temperature was elevated by average 0.31 °C over all vaccine doses (P = 0.0003, 2-way ANOVA). There was no difference between pre- and post-challenge minimum temperatures for scarified animals (P ≥ 0.2, 2-way ANOVAs)
Fig. 4.
Temperature profiles of animals challenged with virulent VACV after vaccination by the scarification (A, C, E) or i.m. (B, D, F) routes. Animals described in Fig. 1 vaccinated at doses of: 1 × 103 ( ); 1 × 104 (
); 1 × 104 ( ); 1 × 105 (
); 1 × 105 ( ); 1 × 106 (
); 1 × 106 ( ); or 1 × 107 (
); or 1 × 107 ( ) pfu/animal, or sham vaccinated with PBS(
) pfu/animal, or sham vaccinated with PBS( ) were challenged i.n. 28 days after vaccination with VACV strain WR at 1 × 107 (A, B), 1 × 108 (C, D), or 3.4 × 108 (E, F) pfu/animal. Animals were monitored daily for temperature for 14 days post-challenge, after which surviving animals were culled. Data is presented as the daily mean temperature for each group. Data from individual mice was subjected to 2-way ANOVAs to compare the two vaccination routes; and separately to compare vaccination/challenge doses for each route with a randomly chosen subset of pre-challenge data from the cognate vaccination dose. Group size = 5.
) were challenged i.n. 28 days after vaccination with VACV strain WR at 1 × 107 (A, B), 1 × 108 (C, D), or 3.4 × 108 (E, F) pfu/animal. Animals were monitored daily for temperature for 14 days post-challenge, after which surviving animals were culled. Data is presented as the daily mean temperature for each group. Data from individual mice was subjected to 2-way ANOVAs to compare the two vaccination routes; and separately to compare vaccination/challenge doses for each route with a randomly chosen subset of pre-challenge data from the cognate vaccination dose. Group size = 5.
For the i.m. route post-challenge maximum temperatures were elevated at all challenge and vaccine doses, by between 0.66 °C and 0.98 °C respective to the post-vaccination maxima (P ≤ 1.1 × 10−10, 2-way ANOVAs). Post-challenge minimum temperatures were also elevated in i.m. vaccinated animals, with differences at 107 and 108 pfu challenge doses (0.38 and 0.39 °C respectively; P ≤ 2.2 × 10−6, 2-way ANOVAs). At the highest challenge dose of 3.4 × 108 pfu, the post-challenge minimum temperature was elevated by 0.2 °C relative to pre-vaccination minima, with P = 0.02, falling between the 95% and 99% confidence limits (2-way ANOVA).
Comparison of post-challenge maximum temperatures between the routes gave a similar picture. Intramuscular vaccinated animals had elevated temperatures relative to scarified animals over all challenge and vaccine doses (0.22 to 0.32 °C, P ranging from 0.01 to 1.5 × 10−5, 2-way ANOVAs). For post-challenge minimum temperatures, i.m. vaccinated mice had values elevated relative to scarified mice only at the lowest challenge dose (P = 4.9 × 10−5, 0.44 °C, 2-way ANOVA). At the higher challenge doses there was no significant difference (P = 0.02 and 0.87, 2-way ANOVA), over all vaccine doses.
In vaccinated mice there were differences in post-challenge disease severity between the two routes when assessed by weight loss (Fig. 5 ). Scarified mice lost more weight after challenge than i.m. vaccinated mice. The difference was observed at all vaccine doses for the mice challenged with 107 or 108 pfu (P = 0.0003 for both, 2-way ANOVA, average percentage difference 3.8% and 2.6% respectively). At the highest challenge dose of 3.4 × 108 pfu there was no difference in weight loss between the routes over all vaccine doses (P = 0.13, 2-way ANOVA).
Fig. 5.
Weight profiles of animals challenged with virulent VACV after vaccination by the scarification (A, C, E) or i.m. (B, D, F) route. Animals described in Fig. 4 vaccinated at doses of: 1 × 103 ( ); 1 × 104 (
); 1 × 104 ( ); 1 × 105 (
); 1 × 105 ( ); 1 × 106 (
); 1 × 106 ( ); or 1 × 107 (
); or 1 × 107 ( ) pfu/animal, or sham vaccinated with PBS(
) pfu/animal, or sham vaccinated with PBS( ) and challenged i.n. with 1 × 107 (A, B), 1 × 108 (C, D), or 3.4 × 108 (E, F) pfu/animal, were monitored daily for weight for 14 days post-challenge, after which surviving animals were culled. Data is presented as the daily mean percentage weight for each group. Percentage weight was calculated as the percentage of initial weight for each animal. Data from individual mice was subjected to 2-wayANOVAs to compare the two vaccination routes; and separately to compare vaccination/challenge doses for each route with a randomly chosen subset of pre-challenge data from the cognate vaccination dose. Group size = 5.
) and challenged i.n. with 1 × 107 (A, B), 1 × 108 (C, D), or 3.4 × 108 (E, F) pfu/animal, were monitored daily for weight for 14 days post-challenge, after which surviving animals were culled. Data is presented as the daily mean percentage weight for each group. Percentage weight was calculated as the percentage of initial weight for each animal. Data from individual mice was subjected to 2-wayANOVAs to compare the two vaccination routes; and separately to compare vaccination/challenge doses for each route with a randomly chosen subset of pre-challenge data from the cognate vaccination dose. Group size = 5.
Intramuscular vaccination leads to depressed temperatures in the post-vaccination period, but elevated temperatures upon subsequent challenge, while the scarification route does not affect temperature either post-vaccination or post-challenge, except at very high challenge dose. However, scarified mice experienced significantly greater weight loss post-challenge than i.m. vaccinated counterparts. At lower challenge doses, weight loss is a more sensitive marker of disease severity than temperature.
Animals were assessed twice daily post-challenge for clinical signs, providing a humane endpoint in addition to weight loss. Most animals exhibited clinical scores, chiefly ruffled fur and occasional hunched posture between days 3–12 (not shown). At vaccine dose 103 pfu, higher clinical scores were observed post-challenge (ruffled fur, hunching, respiratory problems, eye problems, head shaking and head oedema). There were no differences in magnitude of post-challenge clinical score between the two routes at any challenge dose (P ≥ 0.13) (Fig. 6 ). Interestingly, for both routes, clinical scores were lowest in the groups that combined the highest vaccine dose with the highest challenge dose. This suggests the possibility that the larger bolus of virus in the high challenge groups may interact with the adaptive immune response in a manner that improves outcomes, in this system.
Fig. 6.
Post-challenge clinical scores of vaccinated animals. All challenged animals that received vaccine at doses of 1 × 103 ( ); 1 × 104 (
); 1 × 104 ( ); 1 × 105 (
); 1 × 105 ( ); 1 × 106 (
); 1 × 106 ( ); or 1 × 107 (
); or 1 × 107 ( ) pfu/animal, were evaluated daily for clinical signs of disease as described in Methods. The scores for each group over the entire 14 day post-challenge period were aggregated and plotted in histograms, for challenge doses of 1 × 107 (A), 1 × 108 (B), and 3.4 × 108 (C). Comparison of scarification and IM routes was by separate ANOVAs for each challenge dose, over all vaccine doses.
) pfu/animal, were evaluated daily for clinical signs of disease as described in Methods. The scores for each group over the entire 14 day post-challenge period were aggregated and plotted in histograms, for challenge doses of 1 × 107 (A), 1 × 108 (B), and 3.4 × 108 (C). Comparison of scarification and IM routes was by separate ANOVAs for each challenge dose, over all vaccine doses.
4. Discussion
The success of smallpox eradication is testament to both the determination of all those involved, and the efficacy of the live vaccines [26]. However, the live vaccines were associated with sometimes severe or fatal adverse events. The universal open, shedding vaccination lesion could result in vaccine transmission to close contacts, such that contraindicated people are at risk from close contact with others who are recently vaccinated, especially family members.
When vaccination was started by Edward Jenner in the 18th century, scarification was logistically simpler than any type of injection. Thus the early history of successful vaccination in Europe was one of scarification. The precautionary principle provided strong arguments in favour of retaining scarification as the preferred route after parenteral inoculation routes became more generally available. Furthermore, the scarification lesion left a permanent scar identifiable decades later. This provided an easy, low burden method for identifying unvaccinated people in need of vaccination in outbreaks.
There is little data on the efficacy of live smallpox vaccines delivered by the i.m. route, although studies indicate that i.m. vaccination results in lower levels of neutralizing antibody and cellular responses. However there is a notable safety advantage to this route in that it does not generate an open, shedding lesion [15], and significantly reduces risk of transmission to close contacts of vaccinees. It is also more accurate than scarification with regard to the dose delivered. Importantly, previous studies in humans were unable to assess the response of i.m. vaccinees to a serious challenge, for obvious reasons.
Our data show differences in pathology in challenged animals vaccinated by scarification or i.m. routes. VACV is known to suppress the febrile response in mice, through the action of virus encoded immunomodulatory proteins [27]. Poxviruses generally encode a variety of proteins that interact with host cytokines and cytokine receptors (reviewed in: [28], [29], [30], [31]), and route of infection has been shown to affect the composition of leukocyte infiltrates at the infection site [32]. Our data demonstrate differences between i.m. and scarification vaccinated animals after challenge with virulent VACV WR, whereby the febrile response is absent in scarified animals but present in i.m. vaccinated animals. As the challenge route and virus are the same for both groups, this difference is a feature of the vaccination route that affects the response to subsequent severe challenge, and suggests that i.m. vaccinated animals are better able to ablate or avoid virus-encoded mechanisms that suppress innate immunity.
This is supported by our finding that i.m. vaccinated animals were better able to maintain weight after challenge than scarified animals. Weight loss provides a good indicator of eventual mortality [24], and our finding suggests that disease severity in i.m. vaccinated mice after challenge is less than in scarified counterparts.
Although we did not examine the cell-mediated immune response in this study, we did look at development of the antibody response. Scarified animals developed a higher level antibody response than i.m. vaccinated animals with respect to signal strength, and developed a response at lower doses of vaccine. By the measure of specific antibody induction, scarification would appear to be a superior route. However, by the measure of post-challenge disease severity, i.m. vaccination appears to have potential benefits. Clearly there are qualitative differences between the two routes, but these are unlikely to warrant a change in strategy for the general application of smallpox vaccine in an emergency.
Recently, the United States government has acquired a stockpile of live replicating smallpox vaccine for emergency use, and a non-replicating MVA-based vaccine for people who are contraindicated for the live vaccine [23]. It is possible that family members of contraindicated people might also be offered the MVA-based vaccine to remove the risk of live vaccine transmission. However, in an emergency, supplies of the MVA vaccine might be limited, depending on the location of an outbreak(s) and other logistic factors. While there are good reasons for continuing to advocate scarification for general administration of the live vaccine, this study suggests the utility of administering live vaccine by the intramuscular route to family members of contraindicated people if MVA-based vaccine is not available, thus increasing the options for balancing outbreak control with prevention/reduction of adverse events.
The standard dose of smallpox vaccine for humans is ∼1 × 105 to 1 × 106 pfu, and we chose 1 × 105 pfu as the mid-point of our dose range because a previous study has used this dose by the i.m. route in man [15]. Our data show quantitative and qualitative differences between scarification and i.m. routes with respect to disease profile and severity after subsequent challenge. However, i.m. vaccination with live vaccine is still effective in inducing an immune response in mice, and ameliorating disease and preventing death after challenge with VACV WR. We conclude that in an emergency where stockpiles of vaccine(s) are limited, i.m. administration of live smallpox vaccine could be considered for people who are not contraindicated for smallpox vaccination, but who are close contacts of people who are contraindicated; and that further research to examine this is needed.
Acknowledgements
The authors declare that they have no conflicts of interest with the contents of this article. The research was funded by the UK Ministry of Defence.
Content includes material subject to © Crown copyright (2017), Dstl. This material is licensed under the terms of the Open Government Licence except where otherwise stated. To view this licence, visit http://www.nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: psi@nationalarchives.gsi.gov.uk.
References
- 1.Vora S., Damon I., Fulginiti V., Weber S.G., Kahana M., Stein S.L., et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555–1561. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- 2.Lederman E., Groff H., Warkentien T., Reese A., Hruby D., Bolken T., et al. Progressive vaccinia in a military smallpox vaccinee-United States, 2009. Morb Mortal Wkly Rep. 2009;58:532–536. [PubMed] [Google Scholar]
- 3.Lederman E.R., Davidson W., Groff H.L., Smith S.K., Warkentien T., Li Y., et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:1372–1385. doi: 10.1093/infdis/jis510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg R.N., Kennedy J.S. ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin Invest Drugs. 2008;17:555–564. doi: 10.1517/13543784.17.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monath T.P., Caldwell J.R., Mundt W., Fusco J., Johnson C.S., Buller M., et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain) – a second-generation smallpox vaccine for biological defense. Int J Inf Dis. 2004;8:31–44. doi: 10.1016/j.ijid.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrier-Rembert A., Drillien R., Meignier B., Garin D., Crance J.-M. Safety, immunogenicity and protective efficacy in mice of a new cell-cultured Lister smallpox vaccine candidate. Vaccine. 2007;25:8290–8297. doi: 10.1016/j.vaccine.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 7.Mayr A., Stickl H., Müller H., Danner K., Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author's transl) Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene Erste Abteilung Originale Reihe B: Hygiene, Betriebshygiene, praventive Medizin. 1978;167:375–390. [PubMed] [Google Scholar]
- 8.Greenberg R.N., Hay C.M., Stapleton J.T., Marbury T.C., Wagner E., Kreitmeir E., et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Trial Investigating the Safety and Immunogenicity of Modified Vaccinia Ankara Smallpox Vaccine (MVA-BN®) in 56–80-Year-Old Subjects. PloS One. 2016;11:e0157335. doi: 10.1371/journal.pone.0157335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg R.N., Hurley Y., Dinh D.V., Mraz S., Vera J.G., von Bredow D., et al. A Multicenter, Open-Label, Controlled Phase II Study to Evaluate Safety and Immunogenicity of MVA Smallpox Vaccine (IMVAMUNE) in 18–40 Year Old Subjects with Diagnosed Atopic Dermatitis. PloS One. 2015;10:e0138348. doi: 10.1371/journal.pone.0138348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi M., Kimura M., Hirayama M. Report of the National Smallpox Vaccination Research Committee: study of side effects, complications and their treatments. Clin Virol. 1975;3:269–278. [Google Scholar]
- 11.Yokote H., Shinmura Y., Kanehara T., Maruno S., Kuranaga M., Matsui H., et al. Safety of attenuated smallpox vaccine LC16m8 in immunodeficient mice. Clin Vaccine Immunol. 2014;21:1261–1266. doi: 10.1128/CVI.00199-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danon Y.L., Sutter G. Use of the LC16m8 smallpox vaccine in immunocompromised individuals is still too risky. Clinical and Vaccine Immunology. 2015;22:604. doi: 10.1128/CVI.00782-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokote H., Shinmura Y. Reply to “Use of the LC16m8 smallpox vaccine in immunocompromised individuals is still too risky”. Clin Vaccine Immunol. 2015;22:605. doi: 10.1128/CVI.00055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eto A., Saito T., Yokote H., Kurane I., Kanatani Y. Recent advances in the study of live attenuated cell-cultured smallpox vaccine LC16m8. Vaccine. 2015;33:6106–6111. doi: 10.1016/j.vaccine.2015.07.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClain D.J., Harrison S., Yeager C.L., Cruz J., Ennis F.A., Gibbs P., et al. Immunologic responses to vaccinia vaccines administered by different parenteral routes. J Infect Dis. 1997;175:756–763. doi: 10.1086/513968. [DOI] [PubMed] [Google Scholar]
- 16.Coulibaly S., Brühl P., Mayrhofer J., Schmid K., Gerencer M., Falkner F. The nonreplicating smallpox candidate vaccines defective vaccinia Lister (dVV-L) and modified vaccinia Ankara (MVA) elicit robust long-term protection. Virology. 2005;341:91–101. doi: 10.1016/j.virol.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 18.Phelps A., Gates A., Hillier M., Eastaugh L., Ulaeto D. Comparative efficacy of modified vaccinia Ankara (MVA) as a potential replacement smallpox vaccine. Vaccine. 2007;25:34–42. doi: 10.1016/j.vaccine.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Drexler I., Staib C., Kastenmüller W., Stevanović S., Schmidt B., Lemonnier F.A., et al. Identification of vaccinia virus epitope-specific HLA-A* 0201-restricted T cells and comparative analysis of smallpox vaccines. Proc Natl Acad Sci. 2003;100:217–222. doi: 10.1073/pnas.262668999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochstein-Mintzel V., Hänichen T., Huber H., Stickl H. An attenuated strain of vaccinia virus (MVA). Successful intramuscular immunization against vaccinia and variola (author's transl) Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene Erste Abteilung Originale Reihe A: Medizinische Mikrobiologie und Parasitologie. 1974;230:283–297. [PubMed] [Google Scholar]
- 21.Wyatt L.S., Earl P.L., Eller L.A., Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc National Acad Sci USA. 2004;101:4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H., et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79:7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen B.W., Damon I.K., Pertowski C.A., Meaney-Delman D., Guarnizo J.T., Beigi R.H., et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recomm Rep. 2015;64:1–26. [PubMed] [Google Scholar]
- 24.Phelps A., Gates A., Hillier M., Eastaugh L., Ulaeto D. Comparative efficacy of replicating smallpox vaccine strains in a murine challenge model. Vaccine. 2005;23:3500–3507. doi: 10.1016/j.vaccine.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Pulford D., Gates A., Bridge S., Robinson J., Ulaeto D. Differential efficacy of vaccinia virus envelope proteins administered by DNA immunisation in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine. 2004;22:3358–3366. doi: 10.1016/j.vaccine.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Fenner F., Henderson D., Arita I., Jezek Z., Ladnyi I. Switzerland World Health Organization; Geneva: 1988. Smallpox and its eradication. [Google Scholar]
- 27.Alcamí A., Smith G.L. A mechanism for the inhibition of fever by a virus. Proc Natl Acad Sci. 1996;93:11029–11034. doi: 10.1073/pnas.93.20.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahalingam S., Karupiah G. Modulation of chemokines by poxvirus infections. Curr Opin Immunol. 2000;12:409–412. doi: 10.1016/s0952-7915(00)00109-6. [DOI] [PubMed] [Google Scholar]
- 29.Moss B., Shisler J.L. Seminars in immunology. Elsevier; 2001. Immunology 101 at poxvirus U: immune evasion genes; pp. 59–66. [DOI] [PubMed] [Google Scholar]
- 30.Reading P.C., Smith G.L. Vaccinia virus interleukin-18-binding protein promotes virulence by reducing gamma interferon production and natural killer and T-cell activity. J Virol. 2003;77:9960–9968. doi: 10.1128/JVI.77.18.9960-9968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith G.L., Benfield C.T., de Motes C.M., Mazzon M., Ember S.W., Ferguson B.J., et al. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol. 2013;94:2367–2392. doi: 10.1099/vir.0.055921-0. [DOI] [PubMed] [Google Scholar]
- 32.Reading P.C., Smith G.L. A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection. J Gen Virol. 2003;84:1973–1983. doi: 10.1099/vir.0.19285-0. [DOI] [PubMed] [Google Scholar]