Abstract
BACKGROUND
Lifestyle (dietary and/or physical activity [PA]) modification is recommended as first-line therapy to manage polycystic ovary syndrome (PCOS). Current recommendations are based on healthy lifestyle practices for the general public since evidence for unique lifestyle approaches in PCOS is limited and low quality.
OBJECTIVE AND RATIONALE
We aimed to synthesize evidence on dietary and PA behaviors between women with PCOS and those without PCOS. Primary outcomes were overall diet quality, total energy intake and total PA, and secondary outcomes included macronutrients, micronutrients, food groups, foods, glycemic indices, sedentary time and sitting levels. We conducted this work to identify any unique lifestyle behaviors in women with PCOS that could underlie the propensity of weight gain and obesity in PCOS and be targeted for precision nutrition and PA interventions. These findings could be used to inform future practice recommendations and research that more effectively address complications (weight gain, obesity, diabetes, infertility, cardiovascular disease and mental health) in this high-risk population.
SEARCH METHODS
Databases of MEDLINE, Web of Science, Scopus and CINAHL were searched until 15 February 2022 to identify observational studies documenting dietary and PA behaviors between women with PCOS and without PCOS (Controls). Studies on children, adolescents (<18 years), pregnant or menopausal-aged women (>50 years) were excluded. Data were pooled by random-effects models and expressed as (standardized) mean differences (MD) and 95% CIs. The risk of bias was assessed by the Newcastle-Ottawa scale (NOS).
OUTCOMES
Fifty-four studies (N = 39 471 participants; [n = 8736 PCOS; 30 735 Controls]) were eligible (96%; [52/54] NOS scores ≥ 7). Women with PCOS had higher cholesterol (MD: 12.78, 95% CI: 1.48 to 24.08 mg/day; P = 0.03; I2 = 19%), lower magnesium (MD: −21.46, 95% CI: −41.03 to −1.91 mg/day; P = 0.03; I2 = 76%), and a tendency for lower zinc (MD: −1.08, 95% CI: −2.19 to 0.03 mg/day; P = 0.05; I2 = 96%) intake, despite lower alcohol consumption (MD: −0.95, 95% CI: −1.67 to −0.22 g/day; P = 0.02; I2 = 0%) versus Controls. Also, women with PCOS had lower total PA (standardized mean difference: −0.38, 95% CI: −0.72 to −0.03; P = 0.03; I2 = 98%). Conversely, energy, macronutrients (carbohydrate, fat, protein, fiber), micronutrients (folic acid, iron, calcium, sodium), glycemic index and glycemic load were similar (all: P ≥ 0.06). Most eligible studies reported lower total adherence to healthy eating patterns or poorer consumption of major food groups (grains, fruits, vegetables, proteins, seeds, nuts, dairy) in women with PCOS, as described narratively since variable study methodology did not permit meta-analyses.
WIDER IMPLICATIONS
Collective evidence supports that women with PCOS have a lower overall diet quality, poorer dietary intakes (higher cholesterol, lower magnesium and zinc) and lower total PA, despite lower alcohol consumption versus those without PCOS. Considerable heterogeneity among studies reinforces the need for research to address any relative contributions of other factors (e.g. genetic, metabolic or sociodemographic) to the observed differences. These clarifications may contribute to future evidence-based guideline recommendations on monitoring and managing PCOS in the era of precision lifestyle medicine.
Keywords: polycystic ovary syndrome, obesity, lifestyle, nutrition, exercise, reproduction, PCOS
Introduction
Characterized by hyperandrogenism, ovulatory dysfunction and/or polycystic ovarian morphology, polycystic ovary syndrome (PCOS) is a highly heritable, prevalent and complex endocrine disorder affecting up to 18% of reproductive-aged women globally (Carmina and Lobo, 1999; March et al., 2010; Dapas and Dunaif, 2022). Besides reproductive manifestations of increased risk of infertility (Teede et al., 2018) and pregnancy complications (Palomba et al., 2015), women with PCOS often exhibit cardio-metabolic aberrations, including insulin resistance (IR) and compensatory hyperinsulinemia, dyslipidemia and visceral adiposity and are at risk for developing metabolic syndrome, type 2 diabetes and sleep disturbance (Carmina and Lobo, 1999; Wild et al., 2010; Sam and Ehrmann, 2019; Kazemi et al., 2019d, 2020b). Women with PCOS are also at risk for psychosocial comorbidities, including depression, poor self-esteem, anxiety, body image issues, demoralization, social isolation and disordered eating or eating disorders (binge-eating, laxative use, purging, diet pills) (Teede et al., 2010; Naessén et al., 2019; Pirotta et al., 2019; Tay et al., 2019a; Kazemi et al., 2019c).
IR and hyperinsulinemia are key pathophysiological factors in PCOS (Diamanti-Kandarakis and Dunaif, 2012) and have been linked with aggravated hyperandrogenism and reproductive complications. Furthermore, up to 80% of women with PCOS present with overweight or obesity, which further exacerbates inherent IR and compensatory hyperinsulinemia, cardiometabolic and reproductive sequelae (Diamanti-Kandarakis and Dunaif, 2012). Weight management can, therefore, improve PCOS outcomes, in part, through reducing extrinsic IR (Teede et al., 2018). The link between diet and physical activity (PA) as modifiable environmental factors and PCOS complications has biological plausibility (Kazemi et al., 2020a). We and others have shown that adherence to a healthy diet and active lifestyle in women with PCOS improves metabolic, reproductive and psychological outcomes either independent of or in conjunction with, weight loss (Harrison et al., 2011; Kazemi et al., 2018a; Lim et al., 2019; Kazemi et al., 2020a,e). Thus, the International Evidence-based Guideline for the Assessment and Management of PCOS advocates maintaining a healthy weight and preventing weight gain through lifestyle interventions in this clinical population (Teede et al., 2018).
Evidence obtained by us and others supports a propensity for obesity (Barr et al., 2011; Legro et al., 2013; Lin et al., 2019; Kazemi et al., 2021c) and weight gain in women with PCOS during their reproductive years (Teede et al., 2013; Kazemi et al., 2019c, 2021c) and pregnancy (Palomba et al., 2015), that is differentially more pronounced in patients with unhealthy lifestyle behaviors (Awoke et al., 2021). However, whether poorer lifestyle behaviors per se contribute to adiposity and associated PCOS complications have been debated. Specifically, the notion that women with PCOS have a low diet quality, excessive energy intake or engage in shorter PA sessions, is conflicting. Some studies have reported lower overall diet quality (Huijgen et al., 2015; Hosseini et al., 2017; Noormohammadi et al., 2021), increased energy intake (Ahmadi et al., 2013; Eslamian et al., 2017) and decreased PA levels (Moran et al., 2013; Eslamian et al., 2017) in women with versus those without PCOS. Conversely, others have shown higher diet quality (Moran et al., 2017), lower energy intake (Tsai et al., 2013; De Giuseppe et al., 2019) and higher PA levels (Melekoglu et al., 2020) in PCOS cohorts. There is even evidence of similar dietary and PA behaviors between the groups (Wright et al., 2004a; Douglas et al., 2006; Álvarez-Blasco et al., 2011; Cutler et al., 2019). Together, little can be concluded on any difference in dietary or PA behaviors of women with and without PCOS.
An improved understanding of suboptimal dietary and PA behaviors in women with PCOS is crucial for targeted interventions to mediate favorable changes in lifestyle behaviors and body weight. To address this knowledge gap, we conducted a systematic review and meta-analysis to comprehensively characterize and contrast dietary and PA behaviors between women with PCOS and those without PCOS. Our objective was to test the hypothesis that reproductive-aged women with PCOS would exhibit worse dietary and PA behaviors versus their counterparts without PCOS. Our primary outcomes were overall diet quality, energy intake, and total PA levels. As secondary aims, we evaluated whether other dietary and PA factors (micronutrients, macronutrients, food group intake, exercise intensity, leisure activity, sedentary behaviors) differed between groups.
Methods
This systematic review was conducted according to The Cochrane Handbook of Systematic Reviews (Cochrane Handbook for Systematic Reviews of Interventions 2019), and results were reported based on the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) (Stroup et al., 2000). The study protocol was registered at PROSPERO (registration ID: CRD42021252178).
Review question (PEO–Population [P], Exposure [E], Outcome [O])
The PEO criteria are detailed in Supplementary Table SI. Our study question was: in reproductive-aged women (P), do women with PCOS versus their counterparts without PCOS (E) exhibit worse dietary and PA behaviors (O)?
Primary and secondary outcomes
A completed list of study outcomes is presented in Supplementary Table SI and is summarized herein. Our primary outcome was 2-fold for the diet component: overall diet quality and total energy intake; for the PA component, we evaluated total PA levels. Secondary a priori outcomes for diet components included: macronutrients (carbohydrate, fat, protein, fiber, alcohol); micronutrients (folic acid, vitamin D, iron, calcium, magnesium, zinc, sodium); glycemic index [GI]; glycemic load [GL]); and food groups (grains, fruit, vegetable, proteins, seeds and nuts, dairy, added sugar). Regarding PA, our secondary a priori outcomes included exercise intensity (mild, moderate, vigorous), leisure activity levels, and sedentary or sitting levels. Our a priori primary and secondary outcomes were chosen based on evidence from us and others on the clinical relevance of select dietary and PA factors to the pathophysiology and magnitude of signs and symptoms of PCOS, including adiposity (Hahn et al., 2006; Chiu et al., 2018; Kazemi et al., 2018a, 2019a,b,e; Chilibeck et al., 2020; Kazemi et al., 2020a,c,e, 2021a). Additionally, we recorded other post hoc dietary and PA outcomes (e.g. micronutrients and food intakes, engagement in various sports) to assess lifestyle behaviors comprehensively.
Data sources and search strategy
A search for studies published from inception through 26 February 2021 was conducted using MEDLINE, Web of Science, Scopus, and CINAHL databases based on the PEO framework. Subject headings and key terms used in the search strategy for MEDLINE are detailed in Supplementary Table SII. No restrictions (e.g. language, publication year) were imposed. Manual searches of reference lists from included studies supplemented the electronic database searches. Animal studies were excluded. The search was continuously updated up to 15 February 2022.
Inclusion and exclusion criteria
Studies were included if they met the PEO criteria (Supplementary Table SII). Observational studies on reproductive-aged (18–50 years) women in which dietary and PA behaviors were compared between cases (with PCOS) and control groups (without PCOS) were included.
Exclusion criteria included non-peer-reviewed studies; studies without designs of interest (reviews, interventions, case reports, books, reports, conference proceedings, commentaries, letters); duplicated reports from identical studies; non-human models; studies wherein outcomes of interest were not compared between groups; studies on children or adolescents (<18 years), pregnant women, or menopausal-aged women (>50 years); or studies wherein data were irretrievable after contacting their corresponding authors.
Three investigators (C.W., J.D.X. and J.Y.K.) completed the screening processes for inclusion and exclusion of studies independently using the Covidence platform (Covidence.org, Alfred Health, Australia). All disagreements were resolved by a fourth investigator (M.K.).
Data extraction
The following data were extracted: first author’s name, study publication year and country of conduct; participants’ characteristics, including the sample sizes of PCOS and control groups and total sample size, participants’ age and BMI, and racial/ethnic composition (i.e. ancestry) of PCOS and control groups; study design, setting and type of data analysis/collection (prospective/retrospective); dietary and PA assessment tool; reported dietary and PA outcomes; and variables used for matching and/or adjusting of underlying differences (confounders) between groups.
Additional information was also evaluated, including the presence of other (non-PCOS) underlying conditions; tobacco use; medication or supplement use (e.g. metformin, hormonal contraception, multivitamins), or herbs use; PCOS diagnostic criteria applied; and past knowledge of having PCOS where data were available. Where any missing or unclear data was reported, up to two attempts were made to contact the study corresponding author via email to request data or clarify methods. Six investigators (C.T., J.A.G., I.B.X., K.G., J.M. and M.K.) independently completed data extraction. All data extraction was reviewed by M.K.
Quality assessment
The Newcastle-Ottawa scale (NOS) was used to evaluate the methodological quality of studies (Wells et al., 2011), as described in Supplementary Table SIII and our previous work (Kakoly et al., 2018), independently by six investigators (C.W., J.D.X., J.Y.K., I.B.X., K.G. and J.M.). A seventh investigator (M.K.) reviewed all quality assessments and resolved all discrepancies.
Data synthesis and analysis
The effect sizes for each outcome measure were expressed as the weighted mean difference (MD) and 95% CI between cases (women with PCOS) and controls (women without PCOS). Studies were weighted based on the inverse of the variance for the evaluated measure with a random-effects model. Mean differences and SDs of outcome measures were collected to estimate pooled effects for all measures. Where multiple measurement units were used for reporting on specific outcomes (i.e. total carbohydrate, protein, fat [saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs)], PA), we pooled data using standardized mean difference (SMD) to improve statistical power and reported the outcomes in their original measurement units to aid in clinical interpretability.
Chi-square tests were used to evaluate heterogeneity, and Cochran Q and I2 statistics were reported. The I2 value describing the percentage variation between studies was calculated as 100% × ([Q – df])/Q, Q being the χ2 value, and df corresponding to degrees of freedom. Low, moderate and high heterogeneity were defined using the I2 tests (Higgins et al., 2003) and cutoffs of 25%, 50% and 75%, respectively. Tau-square was estimated using the restricted maximum likelihood (REML) method and used to evaluate between-study variance (Higgins et al., 2003).
Sources of heterogeneity were explored using subgroup and sensitivity analyses. We performed a priori subgroup analyses to detect any impact of: age (categorical subgroups: <30 or ≥30 years); BMI (<30 or ≥30 kg/m2); PCOS diagnostic criteria (categorical: 1990 NIH (Zawadski and Dunaif, 1992), 2003 Rotterdam (Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group 2004) or 2006 Androgen Excess and PCOS Society [AE-PCOS] criteria (Azziz et al., 2006), self-reported, unspecified); dietary assessment tool used (24-h dietary recall, food record, food frequency questionnaire, researcher devised surveys); and groups’ country of origin (categorical) on study outcomes where sufficient data were available (≥2 studies per subgroup). We considered subgroup analyses based on the group’s racial/ethnic composition and the tool used to assess PA; however, such analyses were not possible because all but five studies (Douglas et al., 2006; Huijgen et al., 2015; Cutler et al., 2019; Lin et al., 2021; Neubronner et al., 2021) did not report on these data or included predominantly (>60%) White women, and all PA studies used survey data except a single study (Lin et al., 2019) that used accelerometer data.
Further, we performed sensitivity analyses by removing each study and recalculating the overall effect size to determine whether an individual study exerted undue influence (i.e. any alteration in the direction or statistical significance of the overall effect estimate) (Patsopoulos et al., 2008; Iyengar and Greenhouse, 2009; Cochrane Handbook for Systematic Reviews of Interventions 2019). We completed sensitivity analyses to determine the robustness of the observed overall effect estimates and, thus, any assumptions made.
Publication bias was assessed by visual inspection of funnel plots and formal testing by Begg’s rank correlation tests and Egger’s regression asymmetry (Begg and Mazumdar, 1994; Egger et al., 1997). Each funnel plot represents all studies included for each measure; therefore, where data was presented as both SMD and 95% CI for all studies, and MD and 95% CI for certain groups of studies, we provided corresponding funnel plots with SMD data only. M.K. performed all analyses using R version 4.1.0 and RStudio version 1.4.1717 using the meta, metaviz and metacom packages (R Foundation for Statistical Computing, Austria) (Balduzzi et al., 2019). Results were considered significant at P < 0.05.
Results
Literature search
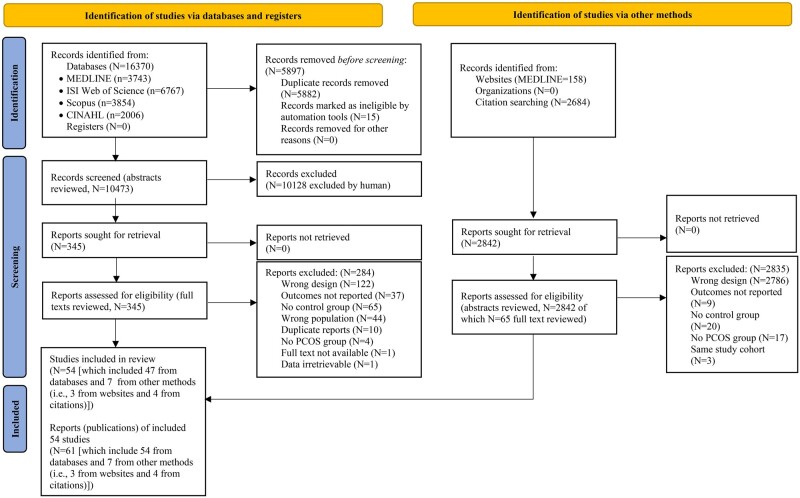
The systematic search resulted in 16 370 records (Fig. 1), of which 54 studies (61 publications) were deemed eligible and included. The selected studies comprised a total of 39 471 participants and 116 experimental arms (n = 8736 PCOS [59 arms]; 30 735 Controls [57 arms]). Reasons for excluding studies at each stage of the literature screening are reported in Fig. 1.
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews, including searches of databases, registers and other sources.
Study characteristics
The general characteristics of the studies are presented in Table I and summarized herein. Studies were published between 2006 and 2022 and were conducted in Iran (Khademi et al., 2010; Pourghassem Gargari et al., 2011; Rajaeieh et al., 2014; Sedighi et al., 2014; Pourghassem Gargari et al., 2015; Shishehgar et al., 2016a,b; Eslamian et al., 2017; Hosseini et al., 2017; Kazemi Jaliseh et al., 2017; Rajaeieh et al., 2018; Zaeemzadeh et al., 2018; Alipour et al., 2019; Shahdadian et al., 2019; Shishehgar et al., 2019; Panjeshahin et al., 2020; Shahrokhi and Naeini, 2020; Badri-Fariman et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Zirak Sharkesh et al., 2021), Italy (Orio et al., 2006; Colombo et al., 2009; Altieri et al., 2013; Barrea et al., 2019), Spain (Álvarez-Blasco et al., 2011; Cutillas-Tolín et al., 2021; Navarro-Lafuente et al., 2022), Australia (Thomson et al., 2009; Moran et al., 2013; Banting et al., 2014; Moran et al., 2015; Copp et al., 2020; Tay et al., 2020), Brazil (Dantas et al., 2015; Cunha et al., 2019), Canada (Cutler et al., 2019), USA (Wright et al., 2004a; Douglas et al., 2006; Lin et al., 2019, 2021), Turkey (Melekoglu et al., 2020), Croatia (Misir et al., 2016), India (Ganie et al., 2019), United Kingdom (Hart et al., 2016), Netherlands (Huijgen et al., 2015; Wang et al., 2021a,b, Poland (Jurewicz et al., 2021; Pokorska-Niewiada et al., 2021; Szczuko et al., 2021), Sweden (Larsson et al., 2016), Austria (Lerchbaum et al., 2021), Taiwan (Tsai et al., 2013) and China (Zhang et al., 2020; Liang et al., 2021; Lu et al., 2021; Wang et al., 2022).
Table I.
General characteristics of the 54 included studies in a comparison of dietary and physical activity behaviors in women with and without PCOS.
| Author, yrs. (reference), and country | Participants' characteristics (n, mean age [yrs.], mean BMI [kg/m2]), PCOS definition, and racial/ethnic composition | Study design, setting, data analysis | Dietary/PA assessment tool | Reported outcomes of interest |
|---|---|---|---|---|
| Alipour et al., 2019, Iran |
|
|
|
|
| Altieri et al., 2013, Italy |
|
|
|
|
| Álvarez-Blasco et al., 2011, Spain |
|
|
|
|
| Badri-Fariman et al., 2021, Iran |
|
|
|
|
| Banting et al., 2014, Australia |
|
|
|
|
| Barrea et al., 2019, Italy |
|
|
|
|
| Colombo et al., 2009, Italy |
|
|
|
|
| Copp et al., 2020, Australia |
|
|
|
|
| Cunha et al., 2019, Brazil |
|
|
|
|
| Cutillas-Tolín et al., 2021, Spain |
|
|
|
|
| Cutler et al., 2019, Canada |
|
|
|
|
| Dantas et al., 2015, Brazil |
|
|
|
|
| Douglas et al., 2006, USA |
|
|
|
|
| Eslamian et al., 2017, Iran |
|
|
|
|
| Ganie et al., 2019, India |
|
|
|
|
| Hart et al., 2016, UK |
|
|
|
|
| Hosseini et al., 2017, Iran |
|
|
|
|
| Huijgen et al., 2015, Netherlands |
|
|
|
|
| Jurewicz et al., 2021, Poland |
|
|
|
|
| Kazemi Jaliseh et al., 2017, Iran |
|
|
|
|
| Khademi et al., 2010, Iran |
|
|
|
|
| Larsson et al., 2016, Sweden |
|
|
|
|
| Lerchbaum et al., 2021, Austria |
|
|
|
|
| Liang et al., 2021, China |
|
|
|
|
| Lin et al., 2019, USA |
|
|
|
|
| Lin et al., 2021, USA |
|
|
|
|
| Lu et al., 2021, China |
|
|
|
|
| Melekoglu et al., 2020, Turkey |
|
|
|
|
| Misir et al., 2016, Croatia |
|
|
|
|
| Moran et al., 2013, 2015, Australia |
|
|
|
|
| Navarro-Lafuente et al., 2022, Spain |
|
|
|
|
| Neubronner et al., 2021, Singapore |
|
|
|
|
| Noormohammadi et al., 2021, Iran |
|
|
|
|
| Orio et al., 2006, Italy |
|
|
|
|
| Panjeshahin et al., 2020, Iran |
|
|
|
|
| Pokorska-Niewiada et al., 2021, Poland |
|
|
|
|
| Pourghassem Gargari et al., 2011, 2015, Iran |
|
|
|
|
| Rajaeieh et al., 2014, 2018, Iran |
|
|
|
|
| Sedighi et al., 2014, Iran |
|
|
|
|
| Shahdadian et al., 2019, Iran |
|
|
|
|
| Shahrokhi and Naeini, 2020, Iran |
|
|
|
|
| Shishehgar et al., 2016a, Iran |
|
|
|
|
| Shishehgar et al., 2019, Iran |
|
|
|
|
| Soodi et al., 2021 and Zirak Sharkesh et al., 2021, Iran |
|
|
|
|
| Szczuko et al., 2021, Poland |
|
|
|
|
| Tay et al., 2020, Australia |
|
|
|
|
| Thara and Divakar, 2017, India |
|
|
|
|
| Thomson et al., 2009, Australia |
|
|
|
|
| Tsai et al., 2013, Taiwan, ROC |
|
|
|
|
| Wang et al., 2021a,b, Netherlands |
|
|
|
|
| Wang et al., 2022, China |
|
|
|
|
| Wright et al., 2004a, USA |
|
|
|
|
| Zaeemzadeh et al., 2018, Iran |
|
|
|
|
| Zhang et al., 2020, China |
|
|
|
|
denote increases in evaluated outcome measures in PCOS compared to control group; ↓denote decreases in evaluated outcome measures in PCOS compared to control group; AEPCOS, Androgen Excess and Polycystic Ovary Syndrome; AHEI, Alternative Healthy Eating Index; ALSWH, Australian Longitudinal Study on Women's Health; AMC, academic medical left; AMDR, Acceptable Macronutrient Distribution Range; aMED, alternate Mediterranean Dietary Score; BMR, basal metabolic rate; CARDIA, Coronary Artery Risk Development in Young Adults; CHO, carbohydrate; Chol, cholesterol; Cu, copper; d, day; DASH, Dietary Approaches to Stop Hypertension; DDS, Dietary Diversity Score; DII, dietary inflammatory index; E, energy; EAT, Eating Attitudes Test; F, fat; Fe, iron; FFM, fat free mass; FFQ, food frequency questionnaire; g, gram; GI, glycemic index; GL, glycemic load; h, hour; HA, hyperandrogenic; HA-OA, hyperandrogenism + oligo/amenorrhea; HEI, Healthy Eating Index; HGI, high glycemic index; hr(s), hour(s); IPAQ, International Physical Activity Questionnaire; IPAQ-SH, International Physical Activity Questionnaire Short Form; K, potassium; kcal, kilocalories; LGI, low glycemic index; LH/FSH, LH/FSH ratio.↔Denote comparable evaluated outcome measures between PCOS and control groups; MED, Mediterranean Diet; MET, metabolic equivalent of task; Mg, magnesium; min, minute; Mn, manganese; mo, month; MUFA, monounsaturated fatty acids; NIH, National Institutes of Health; NR, not-reported; P, protein; PA, physical activity; Ph, phosphorus; PREDIMED, Prevención con Dieta Mediterránea; PUFA, polyunsaturated fatty acids; rMED, relative Mediterranean Dietary Score; Se, selenium; SFA, saturated fatty acids; SSB, sugar-sweetened beverages; TEE, total energy expenditure; Vit, vitamin; wk, week; yrs., years; Zn, zinc.
Most (50/54; 92%) studies were conducted in medical centers, whereas five (six publications) were conducted in community settings (Khademi et al., 2010; Moran et al., 2013; Banting et al., 2014; Moran et al., 2015; Zaimzadeh et al., 2018; Tay et al., 2020). Similarly, most (44/54; 82%) studies had prospective data collection and 10 (12 publications) had retrospective data collection (Pourghassem Gargari et al., 2011; Altieri et al., 2013; Pourghassem Gargari et al., 2015; Hart et al., 2016; Panjeshahin et al., 2020; Badri-Fariman et al., 2021; Lerchbaum et al., 2021; Noormohammadi et al., 2021; Wang et al., 2021a,b; Navarro-Lafuente et al., 2022; Wang et al., 2022).
Of the 54 studies, 49 (91%) had a cross-sectional (Colombo et al., 2009; Thompson et al., 2010; Moran et al., 2013; Banting et al., 2014; Rajaeieh et al., 2014; Moran et al., 2015; Misir et al., 2016; Thara and Divakar, 2017; Rajaeieh et al., 2018; Barrea et al., 2019; Cutler et al., 2019; Shishehgar et al., 2019; Melekoglu et al., 2020; Tay et al., 2020; Zhang et al., 2020; Lerchbaum et al., 2021; Lin et al., 2021; Neubronner et al., 2021; Pokorska-Niewiada et al., 2021; Wang et al., 2021a,b; 2022) or case-control/cohort design (Wright et al., 2004a; Orio et al., 2006; Khademi et al., 2010; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Sedighi et al., 2014; Huijgen et al., 2015; Pourghassem Gargari et al., 2015; Larsson et al., 2016; Shishehgar et al., 2016a; Eslamian et al., 2017; Hosseini et al., 2017; Kazemi Jaliseh et al., 2017; Zaeemzadeh et al., 2018; Alipour et al., 2019; Cunha et al., 2019; Ganie et al., 2019; Lin et al., 2019; Shahdadian et al., 2019; Panjeshahin et al., 2020; Shahrokhi and Naeini, 2020; Badri-Fariman et al., 2021; Cutillas-Tolín et al., 2021; Jurewicz et al., 2021; Liang et al., 2021; Lu et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Szczuko et al., 2021; Zirak Sharkesh et al., 2021; Navarro-Lafuente et al., 2022), 2 had a cohort design (Douglas et al., 2006; Copp et al., 2020) and 3 were observational without specifying their design (Tsai et al., 2013; Dantas et al., 2015; Hart et al., 2016).
Mean age and BMI of participants ranged from 21.0 to 48.2 years and from 19.9 to 35.5 kg/m2, respectively, across PCOS and control arms. Most studies (38/54, 70%) used the Rotterdam criteria for PCOS (Orio et al., 2006; Thomson et al., 2009; Khademi et al., 2010; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Tsai et al., 2013; Sedighi et al., 2014; Dantas et al., 2015; Huijgen et al., 2015; Pourghassem Gargari et al., 2015; Larsson et al., 2016; Misir et al., 2016; Eslamian et al., 2017; Thara and Divakar, 2017; Zaeemzadeh et al., 2018; Alipour et al., 2019; Barrea et al., 2019; Cunha et al., 2019; Cutler et al., 2019; Ganie et al., 2019; Lin et al., 2019; Shahdadian et al., 2019; Shishehgar et al., 2019; Melekoglu et al., 2020; Panjeshahin et al., 2020; Zhang et al., 2020; Badri-Fariman et al., 2021; Cutillas-Tolín et al., 2021; Lerchbaum et al., 2021; Liang et al., 2021; Lu et al., 2021; Neubronner et al., 2021; Noormohammadi et al., 2021; Pokorska-Niewiada et al., 2021; Soodi et al., 2021; Szczuko et al., 2021; Zirak Sharkesh et al., 2021; Wang et al., 2021a,b; Navarro-Lafuente et al., 2022; Wang et al., 2022), whereas five used the National Institutes of Health (NIH) (Wright et al., 2004b; Douglas et al., 2006; Álvarez-Blasco et al., 2011; Kazemi Jaliseh et al., 2017; Lin et al., 2021), and four used the Androgen Excess and Polycystic Ovary Syndrome (AEPCOS) (Colombo et al., 2009; Shishehgar et al., 2016a,b; Hosseini et al., 2017; Jurewicz et al., 2021) criteria. Three did not specify clinical criteria (Rajaeieh et al., 2014; Hart et al., 2016; Rajaeieh et al., 2018; Shahrokhi and Naeini, 2020) and four used self-reported PCOS history (Moran et al., 2013; Banting et al., 2014; Moran et al., 2015; Copp et al., 2020; Tay et al., 2020).
Quality assessment
Supplementary Table SIII shows the NOS quality assessment scores of each study. Most (42/54, 78%) studies had high quality (NOS score ≥ 8), and 12 (24%) were considered poor quality by seven stars (Orio et al., 2006; Colombo et al., 2009; Khademi et al., 2010; Moran et al., 2013; Banting et al., 2014; Rajaeieh et al., 2014; Moran et al., 2015; Hart et al., 2016; Thara and Divakar, 2017; Rajaeieh et al., 2018; Copp et al., 2020; Szczuko et al., 2021) and six stars (Shahrokhi and Naeini, 2020; Tay et al., 2020), respectively, because comparability of their groups were not confirmed by a satisfactory record.
Systematic review
Some outcome measures (diet quality, PA characteristics, food group) were compared qualitatively between groups herein, as pooling analyses were not possible.
Primary outcomes
Thirteen studies (14 publications) (Sedighi et al., 2014; Huijgen et al., 2015; Moran et al., 2015; Hosseini et al., 2017; Barrea et al., 2019; Lin et al., 2019; Panjeshahin et al., 2020; Badri-Fariman et al., 2021; Cutillas-Tolín et al., 2021; Lin et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Zirak Sharkesh et al., 2021; Wang et al., 2022) evaluated diet quality between PCOS and control groups using various indices; thus pooled analyses were impossible. Details of the study groups and characteristics are elaborated in Table I. Most (9/13; 69%) studies reported lower diet quality in PCOS versus Control groups as assessed by lower adherence to the Prevención con Dieta Mediterránea (PREDIMED) score (Barrea et al., 2019), Healthy Eating Index (HEI-2015) (Hosseini et al., 2017), researcher-devised questionnaires (Sedighi et al., 2014), diet diversity score (Soodi et al., 2021; Zirak Sharkesh et al., 2021), higher diet inadequacy using the Preconception Dietary Risk (Huijgen et al., 2015) score, or inflammatory potential of a diet using the dietary inflammatory index (Wang et al., 2022). Across these 13 studies, four showed that lower adherence to the alternative HEI-2010 index (AHEI-2010) (Cutillas-Tolín et al., 2021), lower adherence to the Fertility Diet Score (Chavarro et al., 2007; Noormohammadi et al., 2021), lower adherence to the Mediterranean diet (Wang et al., 2022) and higher adherence to a Western dietary pattern (Badri-Fariman et al., 2021) and high GI and high-fat dietary pattern (Cutillas-Tolín et al., 2021) were associated with an increased likelihood of PCOS. Conversely, a higher adherence to an anti-inflammatory dietary pattern was linked to a lower PCOS risk (Panjeshahin et al., 2020). One study (two publications) (Moran et al., 2013, 2015) showed increased diet quality in women with PCOS, as evidenced by the Mediterranean Diet, dietary glycemic indices, and Dietary Guidelines Index (DGI), while two remaining studies reported comparable HEI-2015 (Lin et al., 2019) and AHEI-2010 (Lin et al., 2021) scores between groups (Table I).
Secondary outcomes
Few studies reported on food groups and used variable measurement indices (e.g. grams, serving numbers or sizes [broadly defined], component scores of dietary indices), making any pooled analyses impossible. Details of these studies are presented in Table I. Overall, women with PCOS exhibited poorer or comparable intakes of major food groups (grains, fruits, vegetables, proteins, seeds and nuts and dairy). Namely, of seven studies reporting on grain intakes, three (Eslamian et al., 2017; Hosseini et al., 2017; Zirak Sharkesh et al., 2021) showed higher refined grains and/or lower whole grains consumption in PCOS versus Control groups, and three (Lin et al., 2019; Badri-Fariman et al., 2021; Lin et al., 2021; Soodi et al., 2021) showed comparable intakes. Of nine studies that reported on total fruit intake, two reported lower (Pourghassem Gargari et al., 2011; Badri-Fariman et al., 2021) and another higher intakes (Hosseini et al., 2017) in PCOS versus Control groups, whereas the remaining six (Altieri et al., 2013; Shishehgar et al., 2016a; Barrea et al., 2019; Lin et al., 2019; Soodi et al., 2021; Wang et al., 2021a) reported comparable higher intake. Of eight studies reporting on total vegetable intakes, three (Shishehgar et al., 2016a; Badri-Fariman et al., 2021; Wang et al., 2021a) showed lower vegetable intakes, and five (Altieri et al., 2013; Hosseini et al., 2017; Barrea et al., 2019; Copp et al., 2020; Zirak Sharkesh et al., 2021) showed similar intakes. Of nine studies reporting on protein food group intake, five showed lower seafood and/or fish intake (Hosseini et al., 2017; Barrea et al., 2019; Badri-Fariman et al., 2021), lower plant protein intake (pulses and/or legumes) (Shishehgar et al., 2016a; Hosseini et al., 2017; Barrea et al., 2019) or increased animal protein intake (Misir et al., 2016) or red, organ, and processed meat (Badri-Fariman et al., 2021), whereas three (Lin et al., 2019, 2021; Soodi et al., 2021) showed comparable intakes of animal or plant proteins. Of three studies reporting on nuts and seeds intakes, two showed a lower proportion of PCOS cohorts who consumed mixed nuts (Badri-Fariman et al., 2021) or tree nuts (Barrea et al., 2019), while another showed similar scores for this food group (Lin et al., 2019). Regarding dairy consumption, eight studies were available, of which three (Pourghassem Gargari et al., 2011; Hosseini et al., 2017; Badri-Fariman et al., 2021) showed lower dairy (total, low fat, whole fat, fermented, processed milk, and/or yogurt, cheese and kefir) intake in PCOS versus Controls, and five showed comparable intakes (Altieri et al., 2013; Rajaeieh et al., 2014; 2018; Lin et al., 2019, 2021; Soodi et al., 2021).
Further, studies also reported on a wide array of other foods and nutrients (e.g. chocolate, coffee, caffeine, tea, wine, beer, ice cream, eggs, sweets with a high GI, vitamins, minerals essential/unsaturated fats or their ratio). These studies reported either lower, higher, or similar intakes between groups, making any conclusions challenging (Table I) (Álvarez-Blasco et al., 2011; Altieri et al., 2013; Larsson et al., 2016; Hosseini et al., 2017; Thara and Divakar, 2017; Zaeemzadeh et al., 2018; Barrea et al., 2019; Cutler et al., 2019; Tay et al., 2020; Lin et al., 2021; Neubronner et al., 2021; Noormohammadi et al., 2021). Likewise, PA characteristics measured by various indices (e.g. percentage of women active/sedentary, step counts and sport, work, or free time) showed poorer, similar or more favorable PA levels in women with PCOS (Álvarez-Blasco et al., 2011; Banting et al., 2014; Huijgen et al., 2015; Misir et al., 2016; Barrea et al., 2019; Cutler et al., 2019; Wang et al., 2022) (Table I).
Meta-analyses
Primary outcomes
Total energy intake
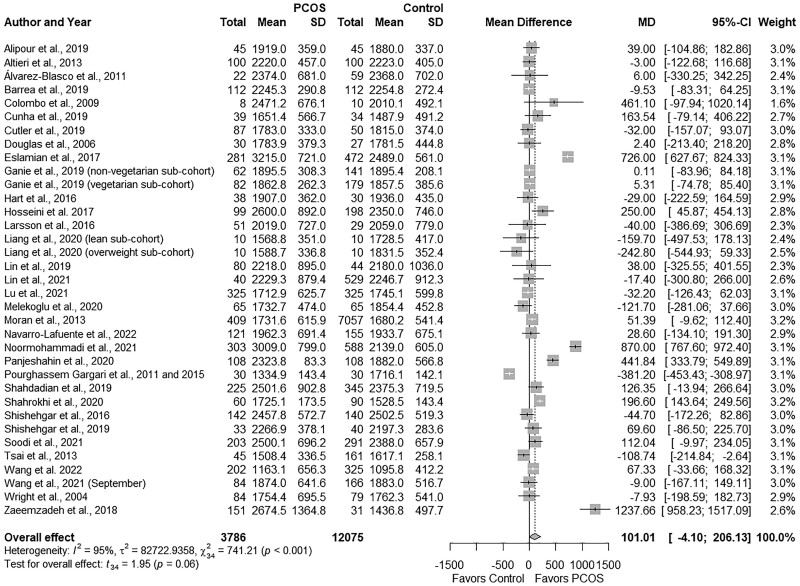
PCOS groups demonstrated comparable total energy intake versus Controls (MD: 101.01, 95% CI: −4.10 to 206.13 kcal/day; P = 0.06; Fig. 2; N = 35) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Moran et al., 2013; Tsai et al., 2013; Pourghassem Gargari et al., 2015; Hart et al., 2016; Larsson et al., 2016; Shishehgar et al., 2016b; Eslamian et al., 2017; Hosseini et al., 2017; Zaeemzadeh et al., 2018; Alipour et al., 2019; Barrea et al., 2019; Cunha et al., 2019; Ganie et al., 2019; Lin et al., 2019; Shahdadian et al., 2019; Shishehgar et al., 2019; Melekoglu et al., 2020; Panjeshahin et al., 2020; Shahrokhi and Naeini, 2020; Liang et al., 2021; Lin et al., 2021; Lu et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Wang et al., 2021a; Navarro-Lafuente et al., 2022; Wang et al., 2022). Studies were highly heterogeneous (I2 = 95%; P < 0.001).
Figure 2.
Forest plot for energy intake in women with and without PCOS with effect estimate expressed as kcal/day. MD, mean difference.
Subgroup analyses based on age, BMI, PCOS criteria, dietary assessment tool or country did not explain heterogeneity, except studies that used the food frequency questionnaire (FFQ), wherein women with PCOS showed higher energy intakes (MD: 278.06, 95% CI: 60.72 to 495.40 kcal/day; P = 0.02) and in studies that were conducted in Iran (MD: −297.75; 95% CI: 17.28 to 578.22 kcal/day; P < 0.01; Fig. 2Supplementary Table SIV). Of note, subgroup analyses were not possible for certain a priori confounders (e.g. self-reported history of PCOS, questionnaire for the assessment of diet quality, or other countries) and, therefore, are not shown in Supplementary Table SIV. Sensitivity analyses showed that excluding two studies (Pourghassem Gargari et al., 2011, 2015; Liang et al., 2021) from the overall effect estimate resulted in significant differences between the groups (all P ≤ 0.04), without changing the direction of effect estimate: ((overweight subgroup in Liang et al. (2021) (MD: 109.77, 95% CI: 3.40 to 216.12 kcal/day) and (Pourghassem Gargari et al., 2011, 2015) (MD: 116.64, 95% CI: −12.82 to 220.45 kcal/day)). We observed no evidence of publication bias (funnel plot, Supplementary Fig. S1; P = 0.15, Begg’s test; P = 0.79, Egger’s test).
Total PA
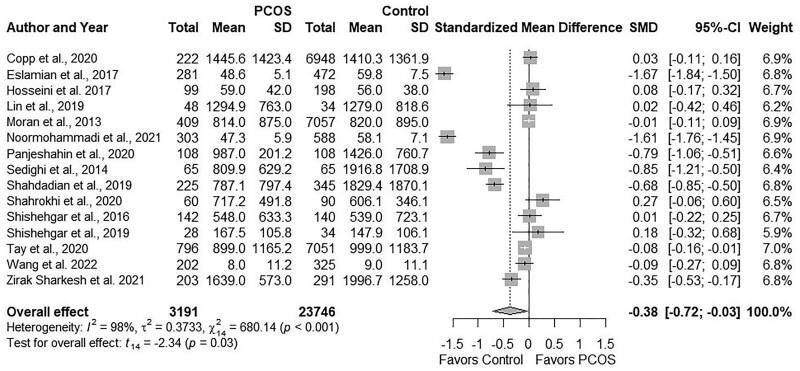
Pooling data for total PA across 15 studies (Moran et al., 2013; Sedighi et al., 2014; Shishehgar et al., 2016b; Eslamian et al., 2017; Hosseini et al., 2017; Lin et al., 2019; Shahdadian et al., 2019; Shishehgar et al., 2019; Copp et al., 2020; Panjeshahin et al., 2020; Shahrokhi and Naeini, 2020; Tay et al., 2020; Noormohammadi et al., 2021; Zirak Sharkesh et al., 2021; Wang et al., 2022) showed comparable levels between groups (SMD: −0.38; 95% CI: −0.72 to −0.03; P = 0.03; Fig. 3). Studies were highly heterogeneous I2 = 98%; P ≤ 0.001). Four studies (Eslamian et al., 2017; Hosseini et al., 2017; Noormohammadi et al., 2021; Wang et al., 2022) reported similar PA in metabolic equivalent (MET)-hour/week (MD: −5.88, 95% CI: −16.69 to 4.92; P = 0.18; Supplementary Fig. S2A), and the remaining 11 (Moran et al., 2013; Sedighi et al., 2014; Shishehgar et al., 2016b; Lin et al., 2019; Shahdadian et al., 2019; Shishehgar et al., 2019; Copp et al., 2020; Panjeshahin et al., 2020; Shahrokhi and Naeini, 2020; Tay et al., 2020; Zirak Sharkesh et al., 2021) reported comparable PA in MET-min/week (MD: −241.89, 95% CI: −524.90 to 41.10; P = 0.09; Supplementary Fig. S2B).
Figure 3.
Forest plot for total physical activity level in women with and without PCOS. SMD, standardized mean difference.
Subgroup analyses based on age, BMI, or country did not explain heterogeneity; however, PA was lower in PCOS versus Controls in analyses of subgroups that used Rotterdam criteria (SMD: −0.79; 95% CI: −1.45 to −0.14; P = 0.03; Supplementary Table SIV). Sensitivity analyses for SMD findings on PA showed that excluding certain studies resulted in no significant differences between groups: (Eslamian et al., 2017) (SMD: −0.28; 95% CI: 0.59 to 0.02); (Noormohammadi et al., 2021) (SMD: −0.29; 95% CI: −0.60 to 0.02); (Panjeshahin et al., 2020) (SMD: −0.35; 95% CI: −0.71 to 0.02); (Sedighi et al., 2014) (SMD: −0.34; 95% CI: −0.71 to 0.02); (Shahdadian et al., 2019) (SMD: −0.35; 95% CI: −0.72 to 0.02; All P = 0.02), albeit the direction of effect estimates was consistent with a lower PA in the PCOS versus Control group. We observed no evidence of publication bias (funnel plot, Supplementary Fig. S3; P = 0.66, Begg’s test; P = 0.47, Egger’s test).
Secondary outcomes
Total carbohydrate and added sugar
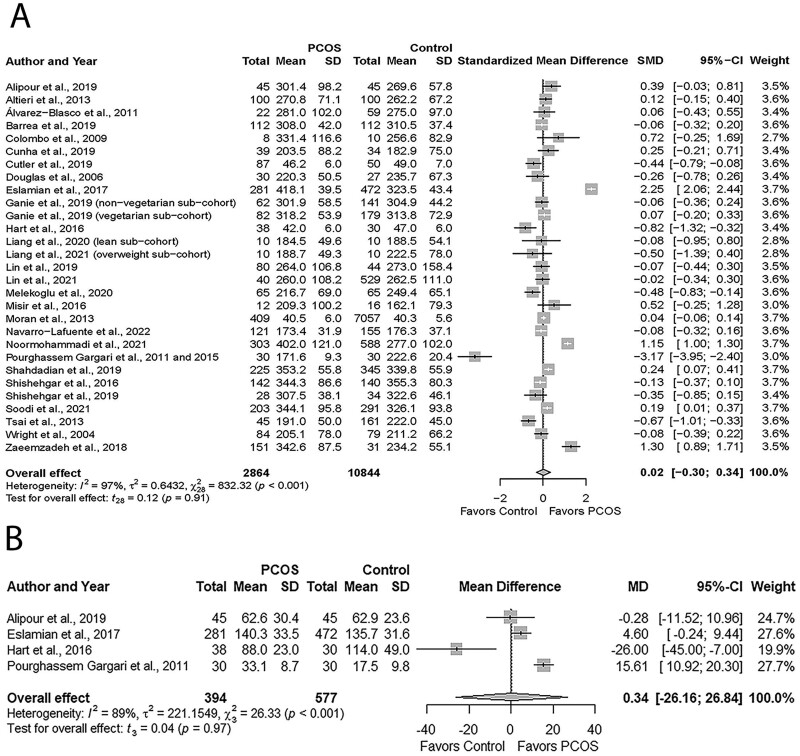
Meta-analysis assessing total carbohydrate intake revealed comparable intakes in women with PCOS versus Controls (SMD: 0.02; 95% CI: −0.30 to 0.34; P = 0.91; Fig. 4; N = 27) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Moran et al., 2013; Tsai et al., 2013; Pourghassem Gargari et al., 2015; Hart et al., 2016; Misir et al., 2016; Shishehgar et al., 2016b; Eslamian et al., 2017; Zaeemzadeh et al., 2018; Alipour et al., 2019; Barrea et al., 2019; Cunha et al., 2019; Cutler et al., 2019; Ganie et al., 2019; Lin et al., 2019; Shahdadian et al., 2019; Shishehgar et al., 2019; Melekoglu et al., 2020; Liang et al., 2021; Lin et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Navarro-Lafuente et al., 2022).
Figure 4.
Forest plots for carbohydrate intake in women with and without PCOS. (A) Total carbohydrate intake. (B) Added sugar expressed in g/day. MD, mean difference; SMD, standardized mean difference.
Carbohydrate intakes were comparable in studies that reported in g/day (MD: 11.85, 95% CI: −6.09 to 29.79; P = 0.19; Supplementary Fig. S4A; N = 24) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Tsai et al., 2013; Pourghassem Gargari et al., 2015; Misir et al., 2016; Shishehgar et al., 2016b; Eslamian et al., 2017; Zaeemzadeh et al., 2018; Alipour et al., 2019; Barrea et al., 2019; Cunha et al., 2019; Ganie et al., 2019; Lin et al., 2019; Shahdadian et al., 2019; Shishehgar et al., 2019; Melekoglu et al., 2020; Liang et al., 2021; Lin et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Navarro-Lafuente et al., 2022) or %energy intake/day (MD: −2.28, 95% CI: −8.82 to 4.27; P = 0.27; Supplementary Fig. S4B; N = 3) (Moran et al., 2013; Hart et al., 2016; Cutler et al., 2019), respectively. Added sugar intakes were similar between groups (MD: 0.34, 95% CI: −26.16 to 26.84 g/day; P = 0.97; Fig. 4B; N = 4) (Pourghassem Gargari et al., 2011; Hart et al., 2016; Eslamian et al., 2017; Alipour et al., 2019). High heterogeneity was identified across studies reporting total carbohydrate and added sugar (all I2 ≤ 89%; All P ≤ 0.001).
Subgroup analyses based on age, BMI, PCOS criteria, dietary assessment tool, or country did not explain heterogeneity (Supplementary Table SIV). Further, the small number of studies (<2) in each subgroup did not allow subgroup analyses for added sugar intake. Sensitivity analyses of SMD results for total carbohydrates showed none of the individual studies influenced the overall effect size. We observed no publication bias for studies reporting carbohydrate intake and added sugar intakes (funnel plot, Supplementary Fig. S5A and B, respectively; All P ≥ 0.14, Begg’s tests; All P ≥ 0.22, Egger’s tests).
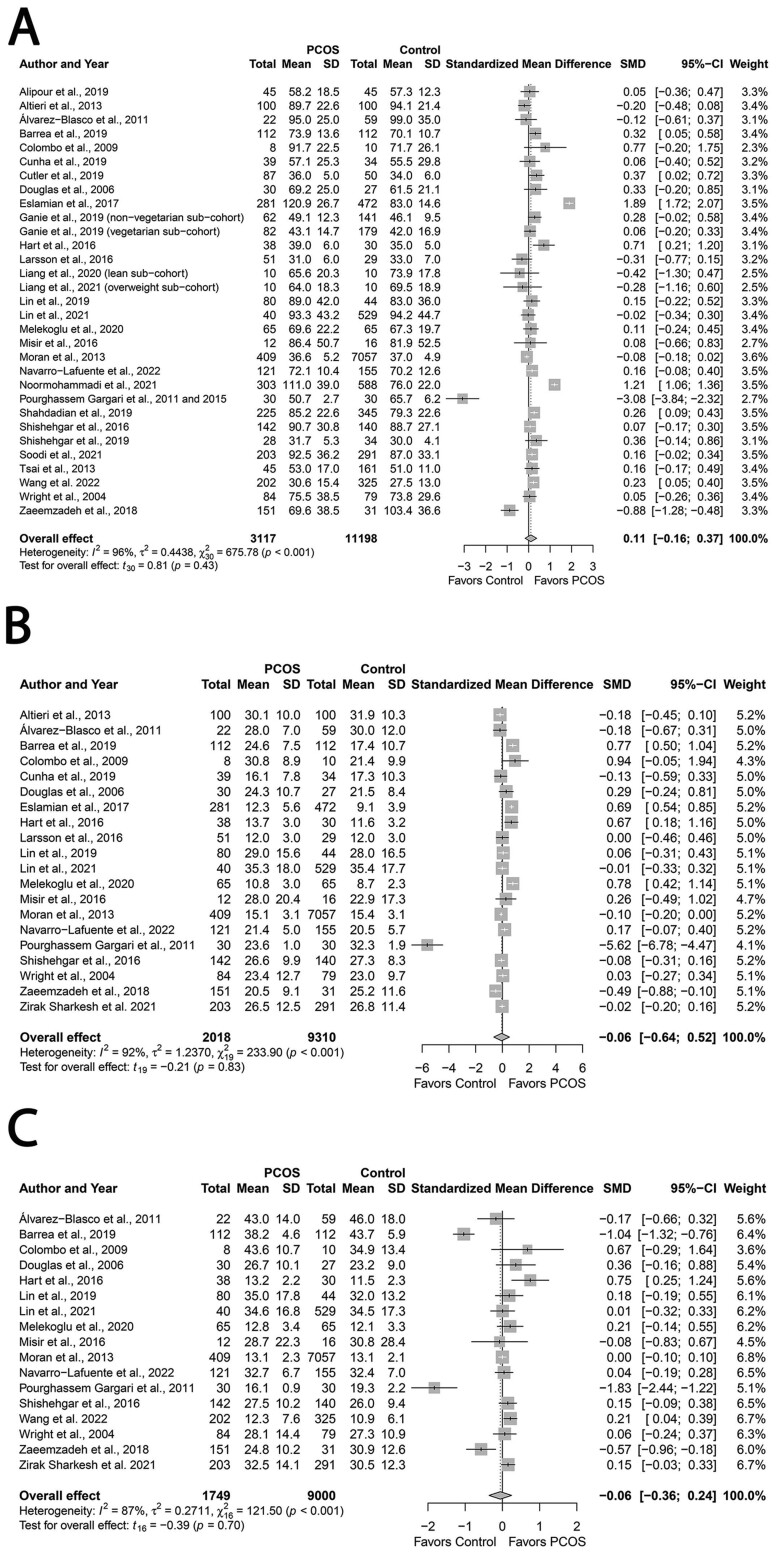
Total fat, SFA, MUFA, PUFA and cholesterol
Total fat consumption was similar between groups. (SMD: 0.11; 95% CI: −0.16 to 0.37; P = 0.43; Fig. 5A; N = 29) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Moran et al., 2013; Tsai et al., 2013; Pourghassem Gargari et al., 2015; Hart et al., 2016; Larsson et al., 2016; Misir et al., 2016; Shishehgar et al., 2016b; Eslamian et al., 2017; Zaeemzadeh et al., 2018; Alipour et al., 2019; Barrea et al., 2019; Cunha et al., 2019; Cutler et al., 2019; Ganie et al., 2019; Lin et al., 2019; Shahdadian et al., 2019; Shishehgar et al., 2019; Melekoglu et al., 2020; Liang et al., 2021; Lin et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Navarro-Lafuente et al., 2022; Wang et al., 2022). Likewise intakes of SFA (SMD: −0.06; 95% CI: −0.64 to 0.52; P = 0.83; Fig. 5B; N = 20) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Moran et al., 2013; Hart et al., 2016; Larsson et al., 2016; Misir et al., 2016; Shishehgar et al., 2016a; Eslamian et al., 2017; Zaeemzadeh et al., 2018; Barrea et al., 2019; Cunha et al., 2019; Lin et al., 2019; Melekoglu et al., 2020; Lin et al., 2021; Zirak Sharkesh et al., 2021; Navarro-Lafuente et al., 2022), MUFA (SMD: −0.06; 95% CI: −0.36; to 0.24; P = 0.70; Fig. 5C; N = 17) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Moran et al., 2013; Hart et al., 2016; Misir et al., 2016; Shishehgar et al., 2016a; Barrea et al., 2019; Lin et al., 2019; Melekoglu et al., 2020; Lin et al., 2021; Zirak Sharkesh et al., 2021; Navarro-Lafuente et al., 2022; Wang et al., 2022) and PUFA (SMD: −0.08; 95% CI: −0.40 to 0.25; P = 0.62; Fig. 5D; N = 17) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Moran et al., 2013; Hart et al., 2016; Misir et al., 2016; Shishehgar et al., 2016a; Barrea et al., 2019; Lin et al., 2019; Melekoglu et al., 2020; Lin et al., 2021; Zirak Sharkesh et al., 2021; Navarro-Lafuente et al., 2022; Wang et al., 2022) were comparable. In contrast, women with PCOS had higher cholesterol intakes (MD: 12.78, 95% CI: 1.48 to 24.08 mg/day; P = 0.03; Fig. 5E; N = 15) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Altieri et al., 2013; Moran et al., 2013; Misir et al., 2016; Cunha et al., 2019; Cutler et al., 2019; Lin et al., 2019; Melekoglu et al., 2020; Lin et al., 2021; Zirak Sharkesh et al., 2021; Navarro-Lafuente et al., 2022; Wang et al., 2022). We observed high heterogeneity across all studies that reported data on fat intake (all I2 ≥ 83%; All P < 0.001), but studies on cholesterol intake were homogeneous (I2 = 18%; P = 0.24).
Figure 5.
Forest plots for fat intake in women with and without PCOS. (A, total fat; B, saturated fatty acids [SFA]; C, monounsaturated fatty acids [MUFA]; D, polyunsaturated fatty acids [PUFA]; E, cholesterol) with cholesterol expressed as mg/day. SMD, standard mean difference.
Figure 5.
Continued
For more clarity, we reported effect estimates for relevant fat intake outcomes separately in g/day and %energy intake/day. Total fat intake in g/day (MD: 3.12, 95% CI: −2.40 to 8.63; P = 0.26; Supplementary Fig. S6A; N = 24) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Tsai et al., 2013; Pourghassem Gargari et al., 2015; Misir et al., 2016; Shishehgar et al., 2016b; Eslamian et al., 2017; Zaeemzadeh et al., 2018; Alipour et al., 2019; Barrea et al., 2019; Cunha et al., 2019; Ganie et al., 2019; Lin et al., 2019; Shahdadian et al., 2019; Melekoglu et al., 2020; Liang et al., 2021; Lin et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Navarro-Lafuente et al., 2022; Wang et al., 2022) and in %energy intake/day (MD: 1.02, 95% CI: −1.70 to 3.74; P = 0.36 Supplementary Fig. S6B; N = 5) (Moran et al., 2013; Hart et al., 2016; Larsson et al., 2016; Cutler et al., 2019; Shishehgar et al., 2019) were comparable between groups.
With respect to SFA, intakes in g/day (MD: 0.11, 95% CI: −2.15 to 2.38 P = 0.92; Supplementary Fig. S6C; N = 16) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Misir et al., 2016; Shishehgar et al., 2016a; Eslamian et al., 2017; Zaeemzadeh et al., 2018; Barrea et al., 2019; Cunha et al., 2019; Lin et al., 2019, 2021; Zirak Sharkesh et al., 2021; Navarro-Lafuente et al., 2022) and in %energy intake/day (MD: 0.92, 95% CI: −1.18 to 3.01; P = 0.26; Supplementary Fig. S6D; N = 4) (Moran et al., 2013; Hart et al., 2016; Larsson et al., 2016; Melekoglu et al., 2020) were similar.
Regarding unsaturated fats, intakes in g/day (MUFA: MD: −0.31, 95% CI: −2.27 to 1.65; P = 0.74; Supplementary Fig. S6E, PUFA: MD: −0.33, 95% CI: −1.41 to 0.76; P = 0.53; Supplementary Fig. S6F, N = 14) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Misir et al., 2016; Shishehgar et al., 2016a; Barrea et al., 2019; Lin et al., 2019, 2021; Zirak Sharkesh et al., 2021; Navarro-Lafuente et al., 2022; Wang et al., 2022) and in %energy intake/day (MUFA: MD: 0.69, 95% CI: −1.46 to 2.85; P = 0.30; Supplementary Fig. S6G; N = 3; PUFA: MD: 0.30, 95% CI: −1.03 to 1.64; P = 0.43; Supplementary Fig. S6H, N = 3) (Moran et al., 2013; Hart et al., 2016; Melekoglu et al., 2020) were comparable.
Subgroup analyses did not explain heterogeneity for SFA, MUFA, and cholesterol. Conversely, PCOS groups demonstrated higher total fat versus Controls in the subgroup of studies that used food records for dietary assessment (SMD: 0.20; 95% CI: 0.02 to 0.38; P = 0.03). We observed higher intakes of PUFA in PCOS versus Controls in the subgroup of studies that used AEPCOS criteria (SMD: 0.17; 95% CI: 0.13 to 0.19; P < 0.01; Supplementary Table SIV). None of the effect estimates for fat intake were sensitive to individual studies except one study: omitting the study by Zirak Sharkesh et al., (2021) resulted in the loss of differences for overall effect estimate in cholesterol intake; however, the direction of effect estimate was retained (MD: 12.10; 95% CI: −0.59 to 24.79; P = 0.06). We observed no evidence of publication bias (funnel plots, Supplementary Fig. S7A–E; All P ≤ 0.16, Begg’s tests; All P ≥ 0.22, Egger’s tests).
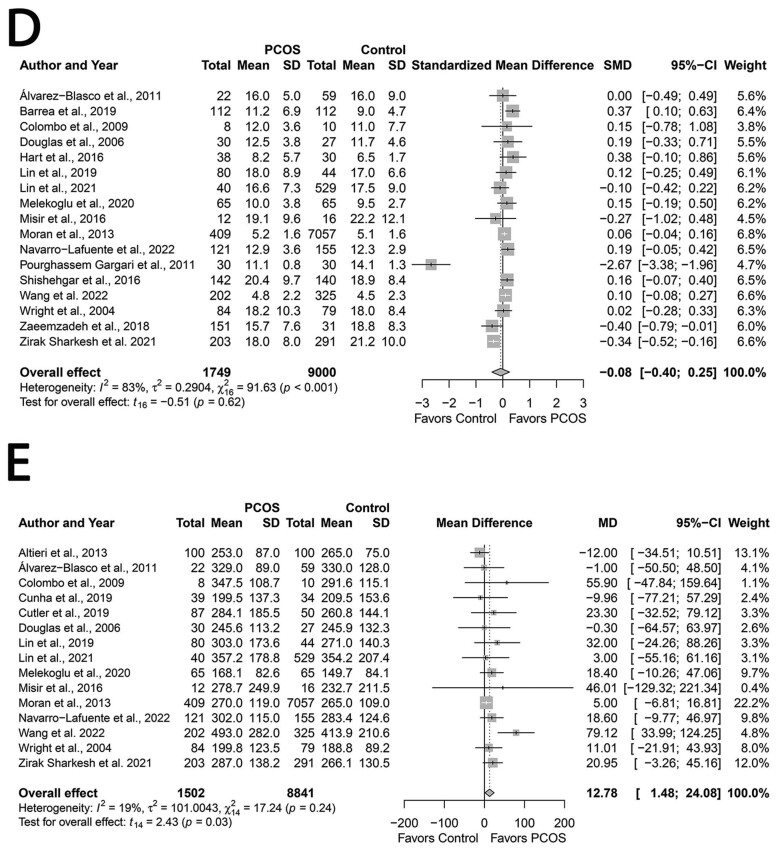
Total protein, fiber and alcohol
Groups had similar total protein intakes (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Moran et al., 2013; Tsai et al., 2013; Pourghassem Gargari et al., 2015; Hart et al., 2016; Larsson et al., 2016; Misir et al., 2016; Shishehgar et al., 2016a; Eslamian et al., 2017; Zaeemzadeh et al., 2018; Alipour et al., 2019; Barrea et al., 2019; Cunha et al., 2019; Cutler et al., 2019; Ganie et al., 2019; Lin et al., 2019; Shahdadian et al., 2019; Shishehgar et al., 2019; Melekoglu et al., 2020; Liang et al., 2021; Lin et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Navarro-Lafuente et al., 2022; Wang et al., 2022) (SMD: −0.04; 95% CI: −0.28 to 0.20; P = 0.72; Fig. 6A; N = 29).
Figure 6.
Forest plots for protein, fiber and alcohol intake in women with and without PCOS. (A) protein intake; (B) fiber intake expressed as g/day; and (C) alcohol intake expressed as g/day. MD, mean difference; SMD, standardized mean difference.
Protein intakes were similar in studies that reported in g/day (MD: −0.26, 95% CI: −3.31 to 2.79; P = 0.86; Supplementary Fig. S8A; N = 24) (Wright et al., 2004a; Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Altieri et al., 2013; Tsai et al., 2013; Pourghassem Gargari et al., 2015; Misir et al., 2016; Shishehgar et al., 2016a; Eslamian et al., 2017; Zaeemzadeh et al., 2018; Alipour et al., 2019; Barrea et al., 2019; Cunha et al., 2019; Ganie et al., 2019; Lin et al., 2019; Shahdadian et al., 2019; Melekoglu et al., 2020; Liang et al., 2021; Lin et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Navarro-Lafuente et al., 2022; Wang et al., 2022) or in %energy intake/day (MD: 0.18, 95% CI: −0.26 to 0.62; P = 0.81; N = 5; Supplementary Fig. S8B) (Moran et al., 2013; Hart et al., 2016; Larsson et al., 2016; Cutler et al., 2019; Shishehgar et al., 2019).
Similarly, pooling analyses showed that PCOS and Control groups had similar intakes of fiber (MD: −1.92, 95% CI: −4.43 to 0.59 g/day; P = 0.13; Fig. 6B; N = 24) (Douglas et al., 2006; Colombo et al., 2009; Álvarez-Blasco et al., 2011; Altieri et al., 2013; Moran et al., 2013; Pourghassem Gargari et al., 2015; Hart et al., 2016; Larsson et al., 2016; Misir et al., 2016; Shishehgar et al., 2016a; Eslamian et al., 2017; Zaeemzadeh et al., 2018; Alipour et al., 2019; Barrea et al., 2019; Cutler et al., 2019; Lin et al., 2019; Shishehgar et al., 2019; Melekoglu et al., 2020; Liang et al., 2021; Noormohammadi et al., 2021; Soodi et al., 2021; Navarro-Lafuente et al., 2022; Wang et al., 2022). In contrast, women with PCOS had lower alcohol intakes (MD: −0.95, 95% CI: −1.67 to −0.22 g/day; P = 0.02; Fig. 6C; N = 5) (Colombo et al., 2009; Álvarez-Blasco et al., 2011; Moran et al., 2013; Lin et al., 2019; Navarro-Lafuente et al., 2022). Studies reporting protein and fiber intakes were heterogeneous (all I2 ≤ 85%; P < 0.001), unlike those reporting alcohol intakes (I2 = 0%; P = 0.71).
Subgroup analyses based on age, BMI, PCOS criteria, dietary assessment tool or country did not explain heterogeneity except lower intakes of fiber in women with PCOS in studies conducted in China (MD: −1.33; 95% CI: −2.58 to −0.09 g/day; P = 0.04) and lower alcohol intakes in the older (≥30 years) subgroup (MD: −0.97; 95% CI: −1.56 to −0.37 g/day; P = 0.03) (Supplementary Table SIV). No study influenced the overall effect size for protein and fiber intakes, revealed by sensitivity analyses. However, removing studies by (Álvarez-Blasco et al., 2011) (MD: −0.99; 95% CI: −2.01 to 0.03 g/day; P = 0.05), (Moran et al., 2013) (MD: −0.96; 95% CI: −2.08 to 0.15 g/day; P = 0.07), and (Navarro-Lafuente et al., 2022) (MD: −0.88; 95% CI: −2.30 to 0.54 g/day; P = 0.14) resulted in the loss of differences in alcohol intakes. We observed no evidence of publication bias for protein, fiber, and alcohol (funnel plots, Supplementary Fig. S9A–C; All P ≥ 0.06, Begg’s tests; All P ≥ 0.11, Egger’s tests).
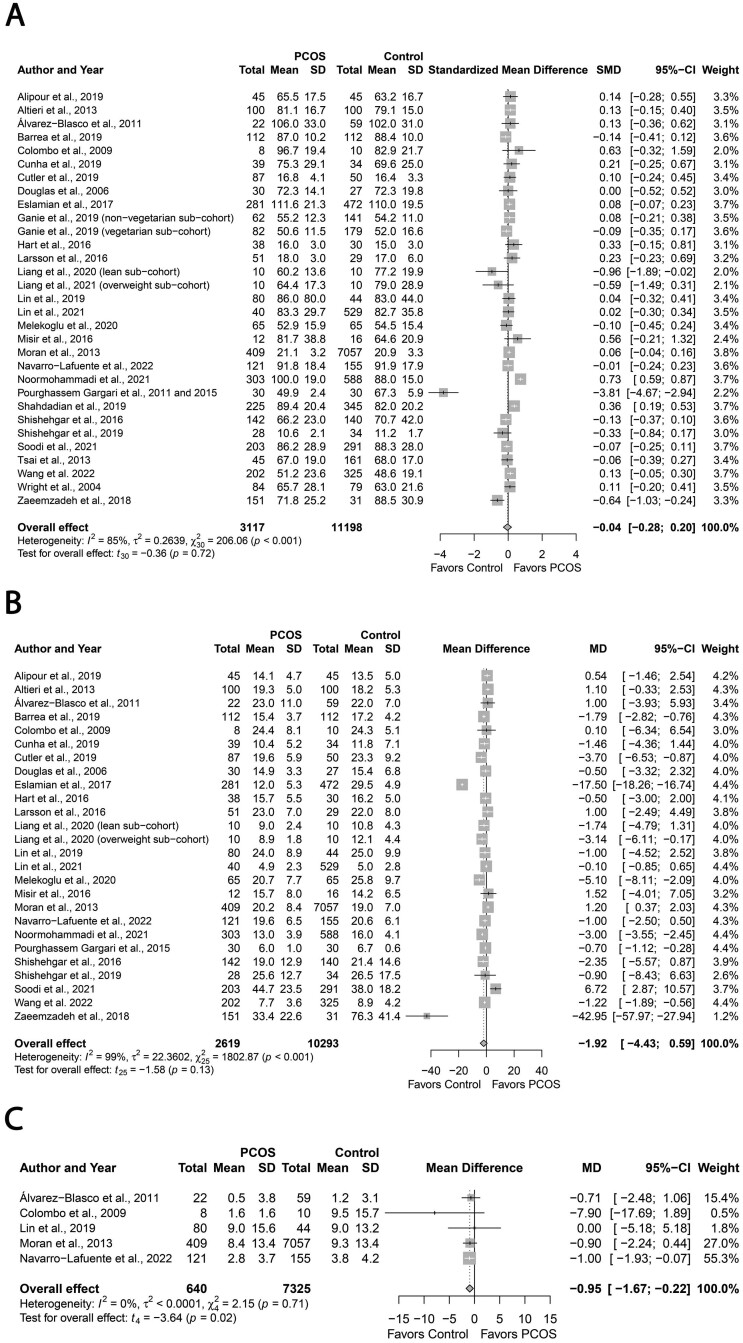
Folic acid, vitamin D, iron, calcium, magnesium, zinc sodium
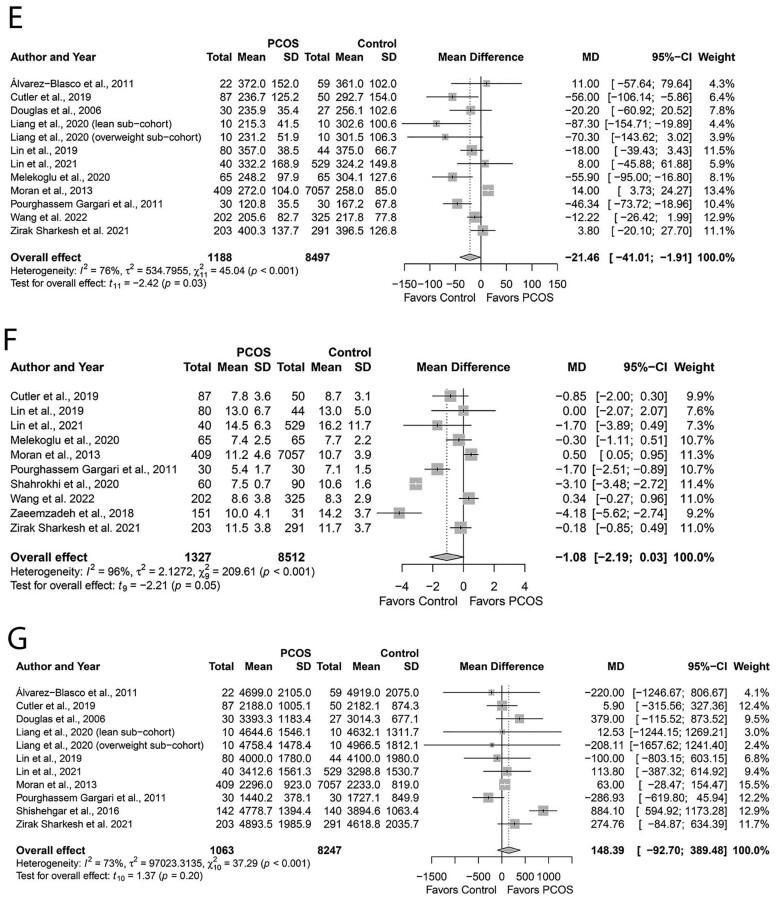
Figure 7A–G demonstrates pooling data for select a priori micronutrient intake. Women with PCOS had comparable intakes of folic acid (MD: −20.80, 95% CI: −42.65 to −1.05 µg/day; P = 0.06; Fig. 7A; N = 9) (Pourghassem Gargari et al., 2011; Moran et al., 2013; Cutler et al., 2019; Lin et al., 2019; Melekoglu et al., 2020; Lin et al., 2021; Szczuko et al., 2021; Zirak Sharkesh et al., 2021; Wang et al., 2022); vitamin D (MD: −1.44, 95% CI: −3.38 to 0.50 µg/day; Fig. 7B; N = 9) (Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Cutler et al., 2019; Lin et al., 2019; Lerchbaum et al., 2021; Liang et al., 2021; Lin et al., 2021; Zirak Sharkesh et al., 2021; Wang et al., 2022); iron (MD: −0.33, 95% CI: −1.45 to 0.80 mg/day; Fig. 7C; N = 7) (Moran et al., 2013; Cutler et al., 2019; Lin et al., 2019; Melekoglu et al., 2020; Lin et al., 2021; Zirak Sharkesh et al., 2021; Wang et al., 2022); calcium (MD: −27.71, 95% CI: −73.47 to 18.05 mg/day; Fig. 7D; N = 8) (Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Moran et al., 2013; Cutler et al., 2019; Lin et al., 2019; Melekoglu et al., 2020; Lin et al., 2021; Soodi et al., 2021); zinc (MD: −1.08, 95% CI: −2.19 to 0.03 mg/day; P = 0.05; Fig. 7F; N = 10) (Pourghassem Gargari et al., 2011; Moran et al., 2013; Zaimzadeh et al., 2018; Cutler et al., 2019; Lin et al., 2019; Melekoglu et al., 2020; Shahrokhi and Naeini, 2020; Lin et al., 2021; Zirak Sharkesh et al., 2021; Wang et al., 2022); or sodium (MD: 148.39, 95% CI: −92.70 to 389.48 mg/day; Fig. 7G; N = 10) (Douglas et al., 2006; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Moran et al., 2013; Shishehgar et al., 2016a; Cutler et al., 2019; Lin et al., 2019; Liang et al., 2021; Lin et al., 2021; Zirak Sharkesh et al., 2021) intakes versus Controls (All P ≥ 0.05). In contrast, women with PCOS exhibited lower magnesium intakes (MD: −21.46, 95% CI: −41.03 to −1.91 mg/day; P = 0.03 Fig. 7E; N = 11) (Douglas et al., 2006; Álvarez-Blasco et al., 2011; Pourghassem Gargari et al., 2011; Moran et al., 2013; Cutler et al., 2019; Lin et al., 2019; Melekoglu et al., 2020; Liang et al., 2021; Lin et al., 2021; Zirak Sharkesh et al., 2021; Wang et al., 2022). There was high heterogeneity across all studies that reported micronutrient intake data (all I2 ≥ 76%; All P < 0.001) except homogenous studies reporting calcium intake (I2 = 36%; P = 0.12).
Figure 7.
Forest plots for micronutrient intake in women with and without PCOS. (A) Folic acid intake expressed as µg/day; (B) vitamin D intake expressed as µg/day; (C) iron intake expressed as mg/day; (D) calcium intake expressed as mg/day; (E) magnesium intake expressed as mg/day; (F) zinc intake expressed as mg/day; and (G) sodium intake expressed as mg/day. MD, mean difference.
Figure 7.
Continued
Subgroup analyses based on age, PCOS criteria, dietary assessment tool, or country did not explain heterogeneity for all micronutrients, except folic acid and magnesium. Lower intakes of folic acid (MD, −41.77; 95% CI: −65.33 to −18.21 µg/day; P = 0.01) and magnesium (MD: −38.98; 95% CI: −69.44 to −8.53 mg/day; P = 0.03) were evident in PCOS versus Control subgroups who were younger (<30 years) and that used Rotterdam criteria. Also, lower intakes of magnesium were evident in the subgroups who were leaner (BMI < 30 kg/m2; MD: −33.33; 95% CI: −63.45 to −3.21 mg/day; P = 0.03) and where dietary intakes were assessed using the 24-hour recalls (MD: −54.44; 95% CI: −78.71 to −30.17 mg/day; P < 0.01). Regarding other dietary assessment tools, PCOS groups showed lower intakes of vitamin D (MD: −0.36; 95% CI: −0.59 to −0.12 µg/day; P = 0.02) and higher intakes of iron (MD: 0.75; 95% CI: 0.49 to 1.01 mg/day; P < 0.01) where FFQ was used (Supplementary Table SIV). None of the individual studies influenced the overall effect size for vitamin D, iron, calcium and sodium, evidenced by sensitivity analyses. In contrast, removing the study by (Moran et al., 2013) (MD: −27.63; 95% CI: −49.58 to −5.67 mg/day; P = 0.02) led to significant differences between groups for folic acid. This observation was similar to removing certain individual studies for zinc intake: ((Moran et al., 2013) (MD: −1.29, 95% CI: −2.46 to −0.11 mg/day; P = 0.04) and (Wang et al., 2022) (MD: −1.26, 95% CI: −2.46 to −0.06 mg/day; P = 0.04). Conversely, removing studies by Cutler et al. (2019) (MD: −18.94, 95% CI: 39.27 to 1.40 mg/day; P = 0.06), Liang et al. (2021) (lean sub-cohort, MD: −17.97, 95% CI: 36.43 to 0.50 mg/day; P = 0.06) (overweight sub-cohort, MD: −19.32, 95% CI: 39.21 to 0.57 mg/day; P = 0.06), Melekoglu et al. (2020) (MD: −17.94, 95% CI: 38.01 to 2.13 mg/day; P = 0.06) and Pourghassem Gargari et al. (2011) (MD: −18.28, 95% CI: 39.15 to 2.60 mg/day; P = 0.06) from magnesium pooled analyses resulted in the loss of differences between groups, albeit the direction of effect estimates remained unchanged. We observed no evidence of publication bias for folic acid, vitamin D, iron and sodium (funnel plot, Supplementary Fig. S10A, B, C and F; all P ≥ 0.45, Begg’s tests; All P ≥ 0.15, Egger’s tests). However, publication bias was detected for calcium and magnesium (funnel plot, Supplementary Fig. 10D and E, respectively) evidenced by the Egger’s test results (All P ≤ 0.02) unlike the Begg’s tests results (all P ≥ 0.53).
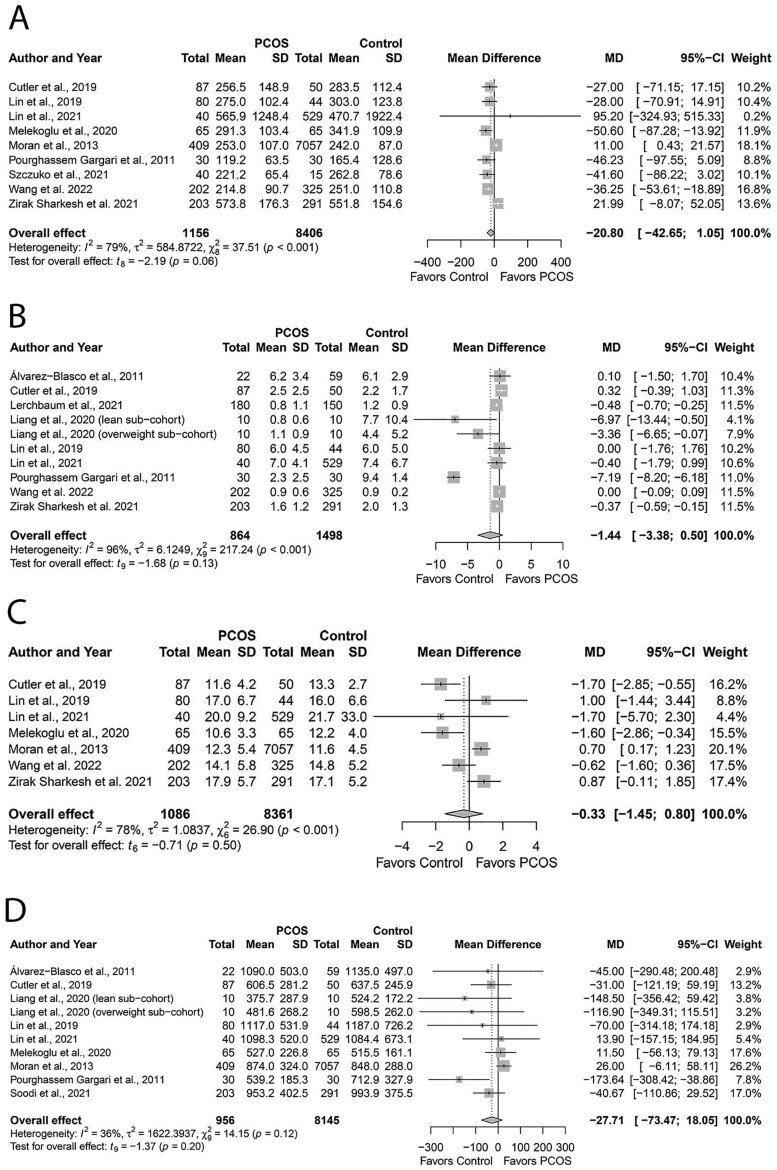
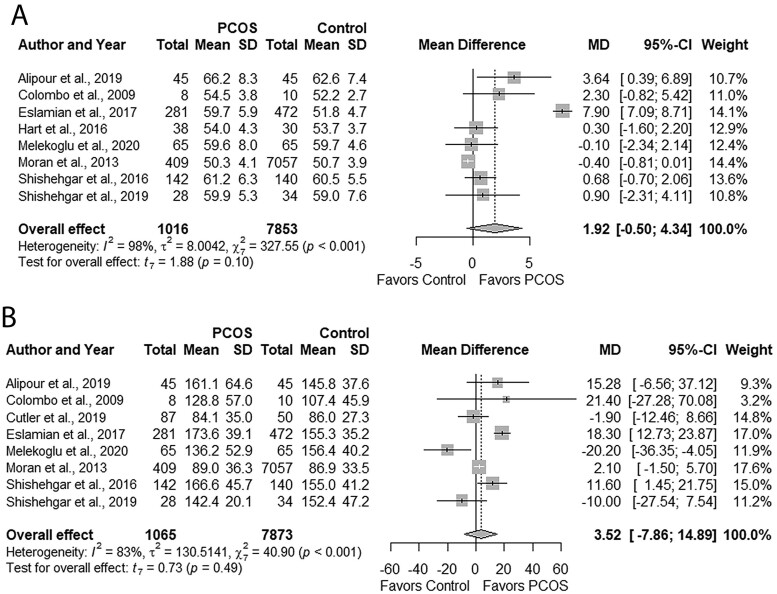
GI and GL
Groups had comparable GI (MD: 1.92, 95% CI: −0.50 to 4.34; P = 0.10; Fig. 8A; N = 8) (Colombo et al., 2009; Moran et al., 2013; Hart et al., 2016; Shishehgar et al., 2016a; Eslamian et al., 2017; Alipour et al., 2019; Shishehgar et al., 2019; Melekoglu et al., 2020) and GL (MD: 3.52, 95% CI: −7.68 to 14.89; P = 0.49; Fig. 8B; N = 8) (Colombo et al., 2009; Moran et al., 2013; Shishehgar et al., 2016a; Eslamian et al., 2017; Alipour et al., 2019; Cutler et al., 2019; Shishehgar et al., 2019; Melekoglu et al., 2020). Studies were moderately heterogeneous (I2 = 41%; P = 0.12).
Figure 8.
Forest plots for status of dietary glycemic indices in women with and without PCOS. (A) glycemic index and (B) glycemic load. MD, mean difference.
Subgroup analyses based on age, PCOS criteria, or dietary assessment tools did not explain heterogeneity (Supplementary Table SIV). Subgroup analyses could not be undertaken for BMI or country due to an insufficient number (<2) of studies. None of the individual studies influenced the overall effect sizes, evidenced by sensitivity analyses. We observed no evidence of publication bias (funnel plots, Supplementary Fig. S11A and B; all P ≥ 0.32, Begg’s test; All P ≥ 0.60, Egger’s test).
Discussion
Main findings
To our knowledge, this is the first systematic review and meta-analysis to synthesize evidence on lifestyle behaviors in women with PCOS. The most significant results of this comprehensive review of 54 observational studies involving 39 471 reproductive-aged women are that women with PCOS exhibit an overall adverse lifestyle behavior, specifically poorer dietary intakes (lower diet quality, higher cholesterol, lower magnesium, tendency for lower zinc), and lower total PA compared to those without PCOS, despite lower alcohol intakes. We also observed worse or similar consumption of core food groups (grains, fruits, vegetables, proteins, seeds, nuts, dairy) for women with PCOS. On subgroup analyses, higher energy and lower fiber intakes were evident in the PCOS groups from select countries, including Iran and China, respectively. Also, lower folic acid intakes were noted in younger (<30 years) PCOS populations. Higher total fat and PUFA intakes were evident in PCOS when food records were used, whereas lower vitamin D and higher iron intakes were observed in PCOS when FFQs were used. Women with PCOS had higher PUFA when PCOS was diagnosed by the AEPCOS criteria. Collectively, these data support the likelihood of poorer lifestyle behaviors in women with PCOS.
Comparison with other studies
Our observations add a novel dimension to current evidence and align with previous systematic reviews and meta-analyses on the relation between poor diet and/or lack of exercise and higher prevalence of chronic disorders with pathophysiologic underpinning similar to PCOS, including gestational diabetes (Mijatovic-Vukas et al., 2018), type 2 diabetes (Lee et al., 2017; Schwingshackl et al., 2018), obesity (de Menezes et al., 2019; Syngelaki et al., 2019) or longitudinal weight gain (Tobias et al., 2015), infertility (Best et al., 2017) and cardiovascular disease (Liyanage et al., 2016). While meta-analyses on micronutrient status in PCOS are sparse, our observations of higher cholesterol and lower magnesium intakes corroborate systematic reviews and meta-analyses reporting higher serum total cholesterol (Wekker et al., 2020) and lower serum magnesium (Babapour et al., 2021) concentrations in PCOS versus Controls. Hypercholesterolemia has been implicated in the development of cardiometabolic and reproductive disruptions, including type 2 diabetes and hyperandrogenemia in PCOS, as elaborated in previous reviews (Wild et al., 2010; Wekker et al., 2020). Magnesium insufficiency has been implicated in the development of IR, or impaired glucose tolerance in PCOS (Babapour et al., 2021), consistent with emerging hypotheses that altered trace mineral status may play a role in PCOS pathogenesis. Furthermore, our observation of lower dietary vitamin D and higher iron intakes in reproductive-aged women with PCOS was consistent with the results of systematic reviews and meta-analyses reporting lower serum vitamin D (Bacopoulou et al., 2017) and higher ferritin (a cellular biomarker of iron storage) (Yin et al., 2020) concentrations in this clinical population. Vitamin D deficiency has been associated with impaired glycemic, hormonal, ovulatory, oxidative and inflammatory status beyond known risks for bone metabolism in PCOS (Nandi et al., 2016; Di Bari et al., 2021; Zhao et al., 2021). Similarly, elevated iron levels in PCOS may be associated with metabolic complications, including IR and adiposity (Yin et al., 2020), albeit data are sparse, warranting further research.
Our results also corroborate those of previous systematic reviews and meta-analyses of no relations between select macronutrients (e.g. total carbohydrate (Liu et al., 2021), total protein (Alhazmi et al., 2012), total fat (Zhu et al., 2019), SFA (de Souza et al., 2015; Zhu et al., 2019), MUFA (Zhu et al., 2019), micronutrients (folic acid (Heinz et al., 2009), calcium (Chung et al., 2016), sodium (Milajerdi et al., 2019), GI (Mulholland et al., 2009; Nagle et al., 2013) or GL (Mulholland et al., 2009; Turati et al., 2019) and chronic disease risk in non-PCOS populations, albeit, contradictory findings exist (Pittas et al., 2007; Meng et al., 2017; Wang et al., 2017; Khan et al., 2018; Zhao et al., 2020)). Small sample sizes and numbers of eligible studies to evaluate some outcome measures (e.g. four studies for added sugar) likely lowered our statistical power to observe real differences. Therefore, further research is needed to confirm our observations for select dietary components.
Explanation of results
The mechanisms through which dietary and PA behaviors may be suboptimal in PCOS are unknown, yet complex interactions between genetic predisposition and environmental determinants may be at play. We and others have attributed an overall lower diet quality, poorer consumption of certain food groups and/or nutrients to physiological (e.g. appetite regulation factors) or psychosocial factors (Robinson et al., 1992; Moran et al., 2004; Barry et al., 2011). Accordingly, we hypothesize that suboptimal lifestyle habits of women with PCOS may be, in part, attributed to disrupting factors including: circadian rhythm (Moore et al., 2021); appetite regulation (Romualdi et al., 2018); energy expenditure (Franks et al., 1996); gut microbiota (Guo et al., 2021); psychosomatic factors (e.g. depression, anxiety) (Barber et al., 2019); lack of education about healthy lifestyle (Steegers-Theunissen et al., 2020); and/or lack of appropriate healthcare access tailored to the needs of this clinical cohort, especially for long-term monitoring (Kazemi et al., 2019c). Presently, the relative contributions of these individual factors to PCOS lifestyle behaviors are unknown, pointing to a persistent research gap.
Strengths and limitations
Strengths of our study include a comprehensive search strategy, extensive outcome measures to assess dietary and PA status, inclusion of a considerable pool of studies and application of conservative statistical methods to interpret findings. We observed no evidence of substantial publication bias in the evaluated outcomes, as evidenced by the Begg’s and Egger’s test results, except for calcium and magnesium intakes. Nevertheless, less symmetric funnel plots observed in select nutrients (e.g. energy, fiber, vitamin D) likely point to publication bias or a difference between studies of higher and lower precision (e.g. small study effects) (Sterne et al., 2011). Our observations had limitations inherent to the sample sizes of some eligible studies and small numbers of studies included for select outcomes. Therefore, our results may be interpreted with caution. The lack of a universal definition for diet quality, dietary and PA assessment tools, and PCOS criteria are also limitations corroborated in previous systematic reviews and meta-analyses of this type (Harrison et al., 2011; Lim et al., 2019; Jalili et al., 2020; Kazemi et al., 2020a,d, 2021a). Failure to account for variations in age, adiposity, lifestyle, socioeconomic status, race and ethnicity, acculturation status, the proportion of PCOS phenotypes across studies, the use of retrospective data or data primarily collected from clinical settings may have contributed to heterogeneity in our observations (Moran et al., 2015; Kazemi et al., 2021a,b,c). Most studies were conducted in medical centers (41/45; 91%) and consisted of cohorts presenting with overweight/obesity (31/43; 73%), indicating our results may be skewed toward severe clinical phenotypes (Ezeh et al., 2013; Kazemi et al., 2019d). Our study was limited by disordered eating or the inability to account for eating disorders that could influence evaluated outcomes. We and others have shown a higher prevalence of disordered eating or eating disorders, including binge-eating disorder, in PCOS (Naessén et al., 2019; Pirotta et al., 2019; Tay et al., 2019b), attributed to obesity, warranting surveillance and management by dietitians and allied health providers (Pirotta et al., 2019). Furthermore, we were unable to compare dietary inositol consumption or supplementation between women with and without PCOS owing to lack of data. However, we recognize a proposed role for inositol (a natural sugar-alcohol) in the management of cardiometabolic and reproductive deregulation in PCOS (Artini et al., 2018; Facchinetti et al., 2020) and note that our findings of poorer intakes of core food groups that contain inositol, including beans, whole grains, nuts, and seeds may signal lower inositol intakes in PCOS.
We observed instability in the significance of the pooled effect estimates with the removal of single studies during sensitivity analyses resulting in the loss of significance for PA, alcohol and magnesium and gaining significance for energy (higher), folic acid and zinc (lower), supporting the need for more research to confirm our observations. Overall, our subgroup results based on established confounders (e.g. age, BMI, dietary and PA assessment tool, PCOS diagnostic criteria, country) did not reveal the sources of heterogeneity across all measures. This was unsurprising given that all women included in the present work were relatively homogenous being of reproductive age (21.0–48.2 years), mostly defined using the Rotterdam criteria (29/45, 64%) and presented with overweight/obesity in medical centers (41/45; 91%), making it challenging to conduct more discrete subgroup analyses (e.g. self-reported definition of PCOS). Nonetheless, our observations of less favorable dietary intakes in certain subgroups, including lower magnesium and folic acid intakes in younger women or higher total fat and PUFA intakes captured by food record tools, may have implications for the dietary management of young at-risk women during their early reproductive stages or highlight the utility of a food record to more accurately capture differences in dietary behaviors (Thompson et al., 2015). Additionally, providing macronutrient intakes using %energy versus g/day may have better reflected individual intakes and subsequently capture any real difference between groups based on acceptable macronutrient distribution range. Furthermore, we considered performing additional subgroup analyses to account for race, ethnicity, previous knowledge of having PCOS, basal metabolic rate, tobacco use, medication use (metformin, hormonal contraceptives), supplements, inflammatory status, reproductive hormones or type of biochemical assays used to measure total testosterone, including liquid chromatography–mass spectrometry. Unfortunately, we lacked sufficient or no data for these analyses, limiting our abilities to understand where real differences lie, which is not uncommon in studies of this type (Gasevic et al., 2015; Kakoly et al., 2018; Rich et al., 2018; Babapour et al., 2021; Hadi et al., 2021). Particularly, reverse causation (improved lifestyle behaviors following PCOS diagnosis) is a significant confounder that has been poorly addressed in PCOS lifestyle research owing to the lack of longitudinal data.
Recommendations for further research
Several questions remain unanswered about which, why, and how dietary and PA behaviors differ in women with PCOS versus Controls, as current evidence on any underlying mechanisms of these differences is sparse and contradictory, making any robust conclusions impossible. Clarifying the role of factors that contribute to adverse lifestyle behaviors in women with PCOS, including abnormalities in appetite regulation or energy expenditure, body composition, genetic and sociodemographic status, with reliable and reproducible tools is needed for both short- and long-term success of lifestyle intervention in this high-risk population (Hoeger et al., 2004; Marsh et al., 2010; Ladson et al., 2011a; Kazemi et al., 2019a,c; 2021c). Filling these knowledge gaps across various reproductive life stages, including puberty, pregnancy, and menopause, and the phenotypic spectrum of PCOS (Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group 2004) with variable degrees of metabolic disturbances and body composition alterations is a priority. Namely, elucidating any suboptimal lifestyle behaviors of pregnant women with PCOS is of particular clinical relevance (Chiu et al., 2018) yet remains poorly studied. Also, research should delineate whether correction of the suboptimal dietary patterns and nutrient intakes and sedentary behaviors identified herein would lead to clinically meaningful improvements in patient-pressing complications, including obesity, infertility and type 2 diabetes in PCOS. These clarifications are critical to elucidate the effectiveness of personalized lifestyle management strategies to improve the cardiometabolic, reproductive, and psychological health of this high-risk cohort. This is in keeping with the emerging era of precision lifestyle medicine in investigating innovative management strategies in other chronic diseases, including type 2 diabetes or cardiovascular disease.
Implications for clinical practice
Our observations have implications for allied healthcare providers (dietitians and exercise physiologists) and physicians to prioritize the identification of suboptimal dietary and PA behaviors in women with PCOS and to guide evidence-based lifestyle management for this prevalent and at-risk population. These findings highlight the importance of early lifestyle intervention at the time of PCOS diagnosis to address modifiable extrinsic factors that can prevent or minimize longitudinal weight gain and associated health complications (Awoke et al., 2021). Provider recommendations should target meeting daily energy intake requirements and adequate consumption of select nutrients (magnesium, vitamin D) and core foods (whole grains, seafood, fish, plant proteins [pulses], nuts, seeds, low-fat dairy) to achieve and maintain optimal health, healthy body weight and prevent long-term weight gain. These recommendations are prudent as we and others have shown PCOS cohorts exhibit poor adherence to energy-restricted diets (Hoeger et al., 2004; Ladson et al., 2011b; Lin et al., 2014; Turner-McGrievy et al., 2014), a propensity for obesity (Kazemi et al., 2018b; Awoke et al., 2021), perception of an inevitability for weight gain (Lin and Lujan, 2014; Lin et al., 2017; Kazemi et al., 2019c), and higher longitudinal weight gain (Teede et al., 2013; Kazemi et al., 2018a; Awoke et al., 2021). Providers may also benefit from improving their ability to work with women with PCOS to improve the lifestyle behaviors of this clinical cohort (Lin et al., 2017; Kazemi et al., 2019c, 2021c).
Conclusion
Collective evidence supports that women with PCOS have lower overall diet quality, poorer dietary intakes (higher cholesterol, lower magnesium and zinc) and lower total PA compared to those without PCOS. Given the observational nature of included studies, we cannot infer causality. Heterogeneity among studies reinforces the need for research to delineate any relative contributions of other factors (genetic, metabolic, sociodemographic) to the observed differences in the era of precision lifestyle medicine. Our findings highlight that providing education on lifestyle modification is crucial for women with PCOS to improve their short- and long-term reproductive, metabolic, and psychological health.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Supplementary Material
Contributor Information
Maryam Kazemi, Division of Nutritional Sciences, Human Metabolic Research Unit, Cornell University, Ithaca, NY, USA; Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes, and Bone Disease, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Joy Y Kim, Division of Nutritional Sciences, Human Metabolic Research Unit, Cornell University, Ithaca, NY, USA.
Cynthia Wan, Division of Nutritional Sciences, Human Metabolic Research Unit, Cornell University, Ithaca, NY, USA.
Julia D Xiong, Division of Nutritional Sciences, Human Metabolic Research Unit, Cornell University, Ithaca, NY, USA.
Julia Michalak, Division of Nutritional Sciences, Human Metabolic Research Unit, Cornell University, Ithaca, NY, USA.
Isabella B Xavier, Division of Nutritional Sciences, Human Metabolic Research Unit, Cornell University, Ithaca, NY, USA.
Kiran Ganga, Division of Nutritional Sciences, Human Metabolic Research Unit, Cornell University, Ithaca, NY, USA.
Chau Thien Tay, Monash Centre for Health Research and Implementation (MCHRI), School of Public Health and Preventive Medicine, Monash University, Clayton, Australia.
Jessica A Grieger, Robinson Research Institute, University of Adelaide, North Adelaide, SA, Australia; Adelaide Medical School, University of Adelaide, Adelaide, SA, Australia.
Stephen A Parry, Cornell Statistical Consulting Unit, Cornell University, Ithaca, NY, USA.
Lisa J Moran, Monash Centre for Health Research and Implementation (MCHRI), School of Public Health and Preventive Medicine, Monash University, Clayton, Australia; Robinson Research Institute, University of Adelaide, North Adelaide, SA, Australia.
Marla E Lujan, Division of Nutritional Sciences, Human Metabolic Research Unit, Cornell University, Ithaca, NY, USA.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding authors.
Authors’ roles
The authors’ contributions were the following: M.K. conceived the topic. M.K. designed the review question, and study with contributions from M.E.L. and L.J.M. M.K. and L.J.M. designed the search strategy and performed searches on the bibliographic databases. C.W., J.D.X., J.Y.K., J.M., I.B.X., K.G. and M.K. screened and reviewed the literature. C.T.T., J.A.G., I.B.X., K.G., J.M., C.W., J.D.X., J.Y.K. and M.K. completed data extraction. C.W., J.D.X., J.Y.K., J.M., I.B.X., K.G. and M.K. conducted the quality assessments of study methods. M.K. resolved all disagreements related to the literature review, data extraction, quality assessment and critical review of outcome data with contributions from M.E.L. and L.J.M. M.K. performed the statistical analyses with contributions from S.A.P. M.K. interpreted the results and wrote the manuscript. All authors reviewed and approved the final version of the manuscript and contributed to the scientific review of study results. The authors are responsible for the study design and conception, data collection and analysis, decision to publish and manuscript preparation. M.K. and M.E.L. supervised the study and had primary responsibility for the final content.
Funding
Funds from the Division of Nutritional Sciences at Cornell University and the National Institutes of Health (Grant No. R01-HD0937848) were used to support the authors (M.E.L. and M.K.) throughout the study period and manuscript preparation. L.J.M. was funded by a National Heart Foundation Future Leader Fellowship. J.A.G. was funded by a National Health and Medical Research Council Ideas Grant (GNT 2000905). M.K. was also funded by the Canadian Institutes of Health Research Postdoctoral Fellowship (No. 459075). The funders had no role in the study design, collection, analyses, interpretation of data, writing of the manuscript or decision to publish.
Conflict of interest
The authors confirm no conflict of interest would confound the proposed study or cause any adverse effect upon past or subsequent interaction with any study participants.
References
- Ahmadi A, Akbarzadeh M, Mohammadi F, Akbari M, Jafari B, Tolide-Ie HR.. Anthropometric characteristics and dietary pattern of women with polycystic ovary syndrome. Indian J Endocrinol Metab 2013;17:672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth BE, Jacobs DR, Leon AS.. Validity and reliability of self-reported physical activity status: the Lipid Research Clinics questionnaire. Med Sci Sports Exerc 1993;25:92–98. [DOI] [PubMed] [Google Scholar]
- Alhazmi A, Stojanovski E, McEvoy M, Garg ML.. Macronutrient intakes and development of type 2 diabetes: a systematic review and meta-analysis of cohort studies. J Am Coll Nutr 2012;31:243–258. [DOI] [PubMed] [Google Scholar]
- Alipour B, Roohelhami E, Shahrdami F, Rashidkhani B.. Dietary glycemic index/glycemic load and their relationship with inflammatory markers in women with polycystic ovary syndrome. Prog Nutr 2019;21:115–121. [Google Scholar]
- Altieri P, Cavazza C, Pasqui F, Morselli AM, Gambineri A, Pasquali R.. Dietary habits and their relationship with hormones and metabolism in overweight and obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2013;78:52–59. [DOI] [PubMed] [Google Scholar]
- Álvarez-Blasco F, Luque-Ramírez M, Escobar-Morreale HF.. Diet composition and physical activity in overweight and obese premenopausal women with or without polycystic ovary syndrome. Gynecol Endocrinol 2011;27:978–981. [DOI] [PubMed] [Google Scholar]
- Artini PG, Obino MER, Sergiampietri C, Pinelli S, Papini F, Casarosa E, Cela V.. PCOS and pregnancy: a review of available therapies to improve the outcome of pregnancy in women with polycystic ovary syndrome. Expert Rev Endocrinol Metab 2018;13:87–98. [DOI] [PubMed] [Google Scholar]
- Asghari G, Rezazadeh A, Hosseini-Esfahani F, Mehrabi Y, Mirmiran P, Azizi F.. Reliability, comparative validity and stability of dietary patterns derived from an FFQ in the Tehran Lipid and Glucose Study. Br J Nutr 2012;108:1109–1117. [DOI] [PubMed] [Google Scholar]
- Awoke MA, Earnest A, Joham AE, Hodge AM, Teede HJ, Brown WJ, Moran LJ.. Weight gain and lifestyle factors in women with and without polycystic ovary syndrome. Hum Reprod 2021;37:129–141. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE. et al. ; Androgen Excess Society. positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237–4245. [DOI] [PubMed] [Google Scholar]
- Babapour M, Mohammadi H, Kazemi M, Hadi A, Rezazadegan M, Askari G.. Associations between serum magnesium concentrations and polycystic ovary syndrome status: a systematic review and meta-analysis. Biol Trace Elem Res 2021;199:1297–1305. [DOI] [PubMed] [Google Scholar]
- Bacopoulou F, Kolias E, Efthymiou V, Antonopoulos CN, Charmandari E.. Vitamin D predictors in polycystic ovary syndrome: a meta-analysis. Eur J Clin Invest 2017;47:746–755. [DOI] [PubMed] [Google Scholar]
- Badri-Fariman M, Naeini AA, Mirzaei K, Moeini A, Hosseini M, Bagheri SE, Daneshi-Maskooni M.. Association between the food security status and dietary patterns with polycystic ovary syndrome (PCOS) in overweight and obese Iranian women: a case-control study. J Ovarian Res 2021;14:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE.. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- Balduzzi S, Rücker G, Schwarzer G.. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banting LK, Gibson-Helm M, Polman R, Teede HJ, Stepto NK.. Physical activity and mental health in women with polycystic ovary syndrome. BMC Womens Health 2014;14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber TM, Hanson P, Weickert MO, Franks S.. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health 2019;13:1179558119874042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr S, Hart K, Reeves S, Sharp K, Jeanes YM.. Habitual dietary intake, eating pattern and physical activity of women with polycystic ovary syndrome. Eur J Clin Nutr 2011;65:1126–1132. [DOI] [PubMed] [Google Scholar]
- Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, de Alteriis G, Tenore GC, Colao A, Savastano S.. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients 2018;10:1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrea L, Arnone A, Annunziata G, Muscogiuri G, Laudisio D, Salzano C, Pugliese G, Colao A, Savastano S.. Adherence to the Mediterranean diet, dietary patterns and body composition in women with polycystic ovary syndrome (PCOS). Nutrients 2019;11:2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JA, Kuczmierczyk AR, Hardiman PJ.. Anxiety and depression in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2011;26:2442–2451. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M.. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed] [Google Scholar]
- Best D, Avenell A, Bhattacharya S.. How effective are weight-loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta-analysis of the evidence. Hum Reprod Update 2017;23:681–705. [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L.. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–469. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Burton NW, Marshall AL, Miller YD.. Reliability and validity of a modified self-administered version of the Active Australia physical activity survey in a sample of mid-age women. Aust N Z J Public Health 2008;32:535–541. [DOI] [PubMed] [Google Scholar]
- Carmina E, Lobo RA.. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab 1999;84:1897–1899. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC.. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur J Clin Nutr 2009;63:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilibeck PD, Kazemi M, McBreairty LE, Zello GA.. Chapter 67: Lifestyle Interventions for Sarcopenic Obesity in Polycystic Ovary Syndrome. Obesity and Diabetes: Scientific Advances and Best Practice. Cham, Switzerland: Springer Nature Switzerland AG; 2020, 907–920. [Google Scholar]
- Chiu YH, Chavarro JE, Souter I.. Diet and female fertility: doctor, what should I eat? Fertil Steril 2018;110:560–569. [DOI] [PubMed] [Google Scholar]
- Chung M, Tang AM, Fu Z, Wang DD, Newberry SJ.. Calcium intake and cardiovascular disease risk. Ann Intern Med 2016;165:856–866. [DOI] [PubMed] [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions. 6.0 edn, 2019. Cochrane.
- Colombo O, Pinelli G, Comelli M, Marchetti P, Sieri S, Brighenti F, Nappi RE, Tagliabue A.. Dietary intakes in infertile women a pilot study. Nutr J 2009;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp T, Cvejic E, McCaffery K, Hersch J, Doust J, Mol BW, Dokras A, Mishra G, Jansen J.. Impact of a diagnosis of polycystic ovary syndrome on diet, physical activity and contraceptive use in young women: findings from the Australian Longitudinal Study of Women's Health. Hum Reprod 2020;35:394–403. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF. et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- Cunha NBD, Ribeiro CT, Silva CM, Rosa ESA, De-Souza DA.. Dietary intake, body composition and metabolic parameters in women with polycystic ovary syndrome. Clin Nutr 2019;38:2342–2348. [DOI] [PubMed] [Google Scholar]
- Cutillas-Tolín A, Arense-Gonzalo JJ, Mendiola J, Adoamnei E, Navarro-Lafuente F, Sánchez-Ferrer ML, Prieto-Sánchez MT, Carmona-Barnosi A, Vioque J, Torres-Cantero AM.. Are dietary indices associated with polycystic ovary syndrome and its phenotypes? A preliminary study. Nutrients 2021;13:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler DA, Pride SM, Cheung AP.. Low intakes of dietary fiber and magnesium are associated with insulin resistance and hyperandrogenism in polycystic ovary syndrome: a cohort study. Food Sci Nutr 2019;7:1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas WS, Marcondes JAM, Shinjo SK, Perandini LA, Zambelli VO, Neves WD, Barcellos CRG, Rocha MP, Yance VDRV, Pereira RTDS. et al. GLUT4 translocation is not impaired after acute exercise in skeletal muscle of women with obesity and polycystic ovary syndrome. Obesity (Silver Spring) 2015;23:2207–2215. [DOI] [PubMed] [Google Scholar]
- Dapas M, Dunaif A.. Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr Rev 2022;bnac001. https://doi.org/10.1210/endrev/bnac001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giuseppe R, Braschi V, Bosoni D, Biino G, Stanford FC, Nappi RE, Cena H.. Dietary underreporting in women affected by polycystic ovary syndrome: a pilot study. Nutr Diet 2019;76:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes EVA, Sampaio HAC, Carioca AAF, Parente NA, Brito FO, Moreira TMM, de Souza ACC, Arruda SPM.. Influence of Paleolithic diet on anthropometric markers in chronic diseases: systematic review and meta-analysis. Nutr J 2019;18:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J. et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015;351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bari F, Catalano A, Bellone F, Martino G, Benvenga S.. Vitamin D, bone metabolism, and fracture risk in polycystic ovary syndrome. Metabolites 2021;11:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Dunaif A.. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012;33:981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas CC, Norris LE, Oster RA, Darnell BE, Azziz R, Gower BA.. Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertil Steril 2006;86:411–417. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani FH, Asghari G, Mirmiran P, Azizi F.. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol 2010;20:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslamian G, Baghestani AR, Eghtesad S, Hekmatdoost A.. Dietary carbohydrate composition is associated with polycystic ovary syndrome: a case-control study. J Hum Nutr Diet 2017;30:90–97. [DOI] [PubMed] [Google Scholar]
- Ezeh U, Yildiz BO, Azziz R.. Referral bias in defining the phenotype and prevalence of obesity in polycystic ovary syndrome. J Clin Endocrinol Metab 2013;98:E1088–E1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchinetti F, Unfer V, Dewailly D, Kamenov ZA, Diamanti-Kandarakis E, Laganà AS, Nestler JE, Soulage CO; Group of ‘Inositol in PCOS and Reproduction’. Inositols in polycystic ovary syndrome: An overview on the advances. Trends Endocrinol Metab 2020;31:435–447. [DOI] [PubMed] [Google Scholar]
- Fjeldsoe BS, Winkler EA, Marshall AL, Eakin EG, Reeves MM.. Active adults recall their physical activity differently to less active adults: test-retest reliability and validity of a physical activity survey. Health Promot J Austr 2013;24:26–31. [DOI] [PubMed] [Google Scholar]
- Franks S, Robinson S, Willis DS.. Nutrition, insulin and polycystic ovary syndrome. Rev Reprod 1996;1:47–53. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J.. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777–781. [DOI] [PubMed] [Google Scholar]
- Ganie MA, Sahar T, Rashid A, Wani IA, Nisar S, Sathyapalan T, Vishnubhatla S, Ramakrishnan L, Parvez T, Geer I.. Comparative evaluation of biomarkers of inflammation among Indian women with polycystic ovary syndrome (PCOS) consuming vegetarian vs. non-vegetarian diet. Front Endocrinol (Lausanne) 2019;10:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasevic D, Ross ES, Lear SA.. Ethnic differences in cardiovascular disease risk factors: a systematic review of North American Evidence. Can J Cardiol 2015;31:1169–1179. [DOI] [PubMed] [Google Scholar]
- Guo J, Shao J, Yang Y, Niu X, Liao J, Zhao Q, Wang D, Li S, Hu J.. Gut Microbiota in Patients with Polycystic Ovary Syndrome: a Systematic Review. Reprod Sci 2022;29:69–83. [DOI] [PubMed] [Google Scholar]
- Hadi A, Arab A, Khalesi S, Rafie N, Kafeshani M, Kazemi M.. Effects of probiotic supplementation on anthropometric and metabolic characteristics in adults with metabolic syndrome: A systematic review and meta-analysis of randomized clinical trials. Clin Nutr 2021;40:4662–4673. [DOI] [PubMed] [Google Scholar]
- Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, Kimmig R, Mann K, Janssen O.. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes 2006;114:577–583. [DOI] [PubMed] [Google Scholar]
- Harrison CL, Lombard CB, Moran LJ, Teede HJ.. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update 2011;17:171–183. [DOI] [PubMed] [Google Scholar]
- Hart K, Barr S, Reeves S, Sharp K, Jeanes Y.. Suboptimal dietary intake is associated with cardiometabolic risk factors in women with polycystic ovary syndrome. Nutr Diet 2016;73:177–183. [Google Scholar]
- Heinz J, Kropf S, Luley C, Dierkes J.. Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: a meta-analysis. Am J Kidney Dis 2009;54:478–489. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS.. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil Steril 2004;82:421–429. [DOI] [PubMed] [Google Scholar]
- Hosseini MS, Dizavi A, Rostami H, Parastouei K, Esfandiari S.. Healthy eating index in women with polycystic ovary syndrome: a case-control study. Int J Reprod Biomed 2017;15:575–582. [PMC free article] [PubMed] [Google Scholar]
- Huijgen NA, Laven JS, Labee CT, Louwers YV, Willemsen SP, Steegers-Theunissen RP.. Are dieting and dietary inadequacy a second hit in the association with polycystic ovary syndrome severity? PLoS One 2015;10:e0142772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Greenhouse J. Sensitivity analysis and diagnostics. In: Cooper H, Hedges LV, Valentine JC. Handbook of Research Synthesis and Meta-Analysis. Russell Sage Foundation: New York, NY, 2009, 417–433.
- Jalili C, Kazemi M, Taheri E, Mohammadi H, Boozari B, Hadi A, Moradi S.. Exposure to heavy metals and the risk of osteopenia or osteoporosis: a systematic review and meta-analysis. Osteoporosis Intl 2020;31:1671–1682. [DOI] [PubMed] [Google Scholar]
- Jurewicz J, Majewska J, Berg A, Owczarek K, Zajdel R, Kaleta D, Wasik A, Rachoń D.. Serum bisphenol A analogues in women diagnosed with the polycystic ovary syndrome—is there an association? Environ Pollut 2021;272:115962. [DOI] [PubMed] [Google Scholar]
- Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, Harrison CL, Ranasinha S, Teede HJ, Moran LJ.. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update 2018;24:455–467. [DOI] [PubMed] [Google Scholar]
- Kazemi Jaliseh H, Ramezani Tehrani F, Behboudi-Gandevani S, Hosseinpanah F, Khalili D, Cheraghi L, Azizi F.. Polycystic ovary syndrome is a risk factor for diabetes and prediabetes in middle-aged but not elderly women: a long-term population-based follow-up study. Fertil Steril 2017;108:1078–1084. [DOI] [PubMed] [Google Scholar]
- Kazemi M, Buddemeyer S, Fassett CM, Gans WM, Johnston KM, Lungu E, Savelle RL, Tolani PN, Dahl WJ.. Chapter 5: pulses and prevention and management of chronic disease. In: Dahl W (ed). Health Benefits of Pulses. Cham, Switzerland: Springer Nature Switzerland AG, 2019a, 55–72. [Google Scholar]
- Kazemi M, Hadi A, Pierson RA, Lujan ME, Zello GA, Chilibeck PD.. Effects of Dietary Glycemic Index and Glycemic Load on Cardiometabolic and Reproductive Profiles in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Adv Nutr 2021;12:161–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M, Jarrett BV, Brink H, Lin A, Hoeger K, Spandorfer S, Lujan M.. Associations between diet quality and ovarian dysmorphology in premenopausal women are mediated by obesity and metabolic aberrations. Proceedings of the American Society for Nutrition, Baltimore, Maryland, USA. June 8-11 (OR36-03-19). Curr Dev Nutr 2019b;3:OR36–03–19. [Google Scholar]
- Kazemi M, Jarrett BY, Parry SA, Thalacker-Mercer A, Hoeger KM, Spandorfer SD, Lujan ME.. Osteosarcopenia in reproductive-aged women with polycystic ovary syndrome: a multicenter case-control study. J Clin Endocrinol Metab 2020b;105:e3400–e3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M, Jarrett BY, Vanden Brink H, Lin AW, Hoeger KM, Spandorfer SD, Lujan ME.. Obesity, insulin resistance, and hyperandrogenism mediate the link between poor diet quality and ovarian dysmorphology in reproductive-aged women. Nutrients 2020c;12:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M, Kim JY, Parry SA, Azziz R, Lujan ME.. Disparities in cardio metabolic risk between Black and White women with polycystic ovary syndrome: a systematic review and meta-analysis. Am J Obstet Gynecol 2021;224:428–444.e8. [DOI] [PubMed] [Google Scholar]
- Kazemi M, Kim JY, Wan C, Xiong JD, Parry SA, Azziz R, Lujan ME.. Comprehensive evaluation of disparities in cardiometabolic and reproductive risk between Hispanic and White women with polycystic ovary syndrome in the United States: a systematic review and meta-analysis. American Journal of Obstetrics and Gynecology 2022;226:187–204.e15. [DOI] [PubMed] [Google Scholar]
- Kazemi M, McBreairty LE, Chilibeck PD, Pierson RA, Chizen DR, Zello GA.. Knowledge, attitudes, and barriers towards dietary pulse consumption in women with polycystic ovary syndrome participating in a multi-disciplinary lifestyle intervention to improve womens health. Sexes 2021c;2:88–103. [Google Scholar]
- Kazemi M, McBreairty LE, Chizen DR, Pierson RA, Chilibeck PD, Zello GA.. A comparison of a pulse-based diet and the Therapeutic Lifestyle Changes diet in combination with exercise and health counselling on the cardio-metabolic risk profile in women with polycystic ovary syndrome: a randomized controlled trial. Nutrients 2018a;10:1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M, McBreairty LE, Chizen DR, Pierson RA, Chilibeck PD, Zello GA.. A Comparison of a pulse-based diet and the therapeutic lifestyle changes diet in combination with exercise and health counselling on the cardio-metabolic risk profile in women with polycystic ovary syndrome: a randomized controlled trial. Nutrients 2018b;10:1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M, McBreairty LE, Zello GA, Pierson RA, Gordon JJ, Serrao SB, Chilibeck PD, Chizen DR.. A pulse-based diet and the Therapeutic Lifestyle Changes diet in combination with health counseling and exercise improve health-related quality of life in women with polycystic ovary syndrome: secondary analysis of a randomized controlled trial. J Psychosom Obstet Gynaecol 2019c;41:144–153. [DOI] [PubMed] [Google Scholar]
- Kazemi M, Pierson RA, Lujan ME, Chilibeck PD, McBreairty LE, Gordon JJ, Serrao SB, Zello GA, Chizen DR.. Comprehensive evaluation of type 2 diabetes and cardiovascular disease risk profiles in reproductive-age women with polycystic ovary syndrome: a large Canadian cohort. J Obstet Gynaecol Can 2019d;41:1453–1460. [DOI] [PubMed] [Google Scholar]
- Kazemi M, Pierson RA, McBreairty LE, Chilibeck PD, Zello GA, Chizen DR.. A randomized controlled trial of a lifestyle intervention with longitudinal follow up on ovarian dysmorphology in women with polycystic ovary syndrome. Clin Endocrinol) 2020e;92:525–535. [DOI] [PubMed] [Google Scholar]
- Kazemi M, Pierson RA, Parry SA, Kaviani M, Chilibeck PD.. Obesity, but not hyperandrogenism or insulin resistance, predicts skeletal muscle mass in reproductive-aged women with polycystic ovary syndrome: a systematic review and meta-analysis of 45 observational studies. Obes Rev 2021a;22:e13255. [DOI] [PubMed] [Google Scholar]
- Kazemi M, Zello GA, McBreairty LE, Pierson RA, Chizen DR, Chilibeck PD.. Sarcopenia is positively associated with poor dietary intakes of women with polycystic ovary syndrome. Appl Physiol Nutr Metab 2019e;44:S26. [Google Scholar]
- Khademi A, Alleyassin A, Aghahosseini M, Tabatabaeefar L, Amini M.. The effect of exercise in PCOS women who exercise regularly. Asian J Sports Med 2010;1:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, Jovanovski E, Ho HVT, Marques ACR, Zurbau A, Mejia SB, Sievenpiper JL, Vuksan V.. The effect of viscous soluble fiber on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 2018;28:3–13. [DOI] [PubMed] [Google Scholar]
- Ladson G, Dodson WC, Sweet SD, Archibong AE, Kunselman AR, Demers LM, Williams NI, Coney P, Legro RS.. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril 2011a;95:1059–1066.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladson G, Dodson WC, Sweet SD, Archibong AE, Kunselman AR, Demers LM, Williams NI, Coney P, Legro RS.. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: A randomized double-blind study. Fertil Steril 2011b;95:1059–1066.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson I, Hulthén L, Landén M, Pålsson E, Janson P, Stener-Victorin E.. Dietary intake, resting energy expenditure, and eating behavior in women with and without polycystic ovary syndrome. Clin Nutr 2016;35:213–218. [DOI] [PubMed] [Google Scholar]
- Lee Y, Park K.. Adherence to a vegetarian diet and diabetes risk: a systematic review and meta-analysis of observational studies. Nutrients 2017;9:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchbaum E, Theiler-Schwetz V, Kollmann M, Wölfler M, Pilz S, Obermayer-Pietsch B, Trummer C.. Effects of vitamin D supplementation on surrogate markers of fertility in PCOS women: a randomized controlled trial. Nutrients 2021;13:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L-m, Rao K-q, Kong L-z, Yao C-h, Xiang H-d, Zhai F-y, Ma G-S, Yang X-g; Technical Working Group of China National Nutrition and Health Survey. A description on the Chinese national nutrition and health survey in 2002. Zhonghua Liu Xing Bing Xue Za Zhi 2005;26:478–484. [PubMed] [Google Scholar]
- Liang Z, Di N, Li L, Yang D.. Gut microbiota alterations reveal potential gut-brain axis changes in polycystic ovary syndrome. J Endocrinol Invest 2021;44:1727–1737. [DOI] [PubMed] [Google Scholar]
- Lim SS, Hutchison SK, Van Ryswyk E, Norman RJ, Teede HJ, Moran LJ.. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2019;3:CD007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AW, Dollahite JS, Sobal J, Lujan ME.. Health-related knowledge, beliefs and self-efficacy in women with polycystic ovary syndrome. Hum Reprod 2018;33:91–100. [DOI] [PubMed] [Google Scholar]
- Lin AW, Kazemi M, Jarrett BY, Vanden Brink H, Hoeger KM, Spandorfer SD, Lujan ME.. Dietary and physical activity behaviors in women with polycystic ovary syndrome per the new International Evidence-Based Guideline. Nutrients 2019;11:2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AW, Lujan ME.. Comparison of dietary intake and physical activity between women with and without polycystic ovary syndrome: a review. Adv Nutr 2014;5:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AW, Siscovick D, Sternfeld B, Schreiner P, Lewis CE, Wang ET, Merkin SS, Wellons M, Steffen L, Calderon-Margalit R, Cassano PA, Lujan ME.. Associations of diet, physical activity and polycystic ovary syndrome in the Coronary Artery Risk Development in Young Adults Women's Study. BMC Public Health 2021;21:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YS, Wu QJ, Lv JL, Jiang YT, Sun H, Xia Y, Chang Q, Zhao YH.. Dietary carbohydrate and diverse health outcomes: umbrella review of 30 systematic reviews and meta-analyses of 281 observational studies. Front Nutr 2021;8:670411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage T, Ninomiya T, Wang A, Neal B, Jun M, Wong MG, Jardine M, Hillis GS, Perkovic V.. Effects of the Mediterranean Diet on cardiovascular outcomes—a systematic review and meta-analysis. PLoS One 2016;11:e0159252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Li X, Lv L, Xu Y, Wu B, Huang C.. Dietary and serum n-3 PUFA and polycystic ovary syndrome: a matched case-control study. Br J Nutr 2021;10:1–10. [DOI] [PubMed] [Google Scholar]
- March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ.. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010;25:544–551. [DOI] [PubMed] [Google Scholar]
- Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC.. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr 2010;92:83–92. [DOI] [PubMed] [Google Scholar]
- Martínez-González MA, García-Arellano A, Toledo E, Salas-Salvadó J, Buil-Cosiales P, Corella D, Covas MI, Schröder H, Arós F, Gómez-Gracia E. et al. ; PREDIMED Study Investigators. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One 2012;7:e43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melekoglu E, Goksuluk D, Akal Yildiz E.. Association between dietary glycaemic index and glycaemic load and adiposity indices in polycystic ovary syndrome. J Am Coll Nutr 2020;39:537–546. [DOI] [PubMed] [Google Scholar]
- Meng Y, Bai H, Wang S, Li Z, Wang Q, Chen L.. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2017;131:124–131. [DOI] [PubMed] [Google Scholar]
- Meyer A-M, Evenson KR, Morimoto L, Siscovick D, White E.. Test-retest reliability of the Women's Health Initiative physical activity questionnaire. Med Sci Sports Exerc 2009;41:530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic-Vukas J, Capling L, Cheng S, Stamatakis E, Louie J, Cheung NW, Markovic T, Ross G, Senior A, Brand-Miller JC. et al. Associations of diet and physical activity with risk for gestational diabetes mellitus: a systematic review and meta-analysis. Nutrients 2018;10:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milajerdi A, Djafarian K, Shab-Bidar S.. Dose–response association of dietary sodium intake with all-cause and cardiovascular mortality: a systematic review and meta-analysis of prospective studies. Public Health Nutr 2019;22:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F.. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr 2010;13:654–662. [DOI] [PubMed] [Google Scholar]
- Misir A, Banjari I, Loncar I.. Comparison of diet in women of reproductive age with and without diagnosed polycystic ovary syndrome—pilot study. Med Pregl 2016;69:274–280. [DOI] [PubMed] [Google Scholar]
- Momenan AA, Delshad M, Sarbazi NR, Ghaleh N, Ghanbarian A, Azizi F.. Reliability and validity of the Modifiable Activity Questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med 2012;15:279–282. [PubMed] [Google Scholar]
- Moore JM, Waldrop SW, Cree-Green M.. Weight management in adolescents with polycystic ovary syndrome. Curr Obes Rep 2021;10:311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran L, Grieger J, Mishra G, Teede H.. The association of a Mediterranean-style diet pattern with polycystic ovary syndrome status in a community cohort study. Nutrients 2015;7:8553–8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LJ, Brown WJ, McNaughton SA, Joham AE, HJ T.. Weight management practices associated with PCOS and their relationships with diet and physical activity. Hum Reprod 2017;32:669–678. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Noakes M, Clifton PM, Wittert GA, Tomlinson L, Galletly C, Luscombe ND, Norman RJ.. Ghrelin and measures of satiety are altered in polycystic ovary syndrome but not differentially affected by diet composition. J Clin Endocrinol Metab 2004;89:3337–3344. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Ranasinha S, Zoungas S, McNaughton SA, Brown WJ, Teede HJ.. The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Hum Reprod 2013;28:2276–2283. [DOI] [PubMed] [Google Scholar]
- Mulholland HG, Murray LJ, Cardwell CR, Cantwell MM.. Glycemic index, glycemic load, and risk of digestive tract neoplasms: a systematic review and meta-analysis. Am J Clin Nutr 2009;89:568–576. [DOI] [PubMed] [Google Scholar]
- Naessén S, Söderqvist G, Carlström K.. So similar and so different: circulating androgens and androgen origin in bulimic women. J Steroid Biochem Mol Biol 2019;185:184–188. [DOI] [PubMed] [Google Scholar]
- Nagle CM, Olsen CM, Ibiebele TI, Spurdle AB, Webb PM; Astralian Ovarian Cancer Study Group. Glycemic index, glycemic load and endometrial cancer risk: results from the Australian National Endometrial Cancer study and an updated systematic review and meta-analysis. Eur J Nutr 2013;52:705–715. [DOI] [PubMed] [Google Scholar]
- Nandi A, Sinha N, Ong E, Sonmez H, Poretsky L.. Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Investig 2016;25:15–28. [DOI] [PubMed] [Google Scholar]
- Navarro-Lafuente F, Arense-Gonzalo JJ, Sánchez-Ferrer ML, Prieto-Sánchez MT, Cutillas-Tolín A, Mendiola J, Adoamnei E, Gazabat-Barbado E, Vioque J, Torres-Cantero AM.. Fat intake pattern in women with polycystic ovary syndrome. Reprod Biomed Online 2022;44:93–103. [DOI] [PubMed] [Google Scholar]
- Neubronner SA, Indran IR, Chan YH, Thu AWP, Yong EL.. Effect of body mass index (BMI) on phenotypic features of polycystic ovary syndrome (PCOS) in Singapore women: a prospective cross-sectional study. BMC Womens Health 2021;21:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noormohammadi M, Eslamian G, Malek S, Shoaibinobarian N, Mirmohammadali SN.. The association between fertility diet score and polycystic ovary syndrome: a case-control study. Health Care Women Int 2021;43:1–15. [DOI] [PubMed] [Google Scholar]
- Orio F Jr, Giallauria F, Palomba S, Cascella T, Manguso F, Vuolo L, Russo T, Tolino A, Lombardi G, Colao A. et al. Cardiopulmonary impairment in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91:2967–2971. [DOI] [PubMed] [Google Scholar]
- Pala V, Sieri S, Palli D, Salvini S, Berrino F, Bellegotti M, Frasca G, Tumino R, Sacerdote C, Fiorini L. et al. Diet in the Italian EPIC cohorts: presentation of data and methodological issues. Tumori 2003;89:594–607. [DOI] [PubMed] [Google Scholar]
- Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC.. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015;21:575–592. [DOI] [PubMed] [Google Scholar]
- Panjeshahin A, Salehi-Abargouei A, Anari AG, Mohammadi M, Hosseinzadeh M.. Association between empirically derived dietary patterns and polycystic ovary syndrome: a case-control study. Nutrition 2020;79–80:110987. [DOI] [PubMed] [Google Scholar]
- Patsopoulos NA, Evangelou E, Ioannidis JP.. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 2008;37:1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippaerts RM, Westerterp KR, Lefevre J.. Doubly labelled water validation of three physical activity questionnaires. Int J Sports Med 1999;20:284–289. [DOI] [PubMed] [Google Scholar]
- Pirotta S, Barillaro M, Brennan L, Grassi A, Jeanes YM, Joham AE, Kulkarni J, Couch LM, Lim SS, Moran LJ.. Disordered eating behaviours and eating disorders in women in Australia with and without polycystic ovary syndrome: a cross-sectional study. JCM 2019;8:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F.. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol 1997;26(Suppl 1):S152–S160. [DOI] [PubMed] [Google Scholar]
- Pittas AG, Lau J, Hu FB, Dawson-Hughes B.. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorska-Niewiada K, Brodowska A, Szczuko M.. The content of minerals in the PCOS group and the correlation with the parameters of metabolism. Nutrients 2021;13:2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourghassem Gargari B, Houjeghani S, Farzadi L, Houjeghani S, Safaeiyan A.. Relationship between serum leptin, ghrelin and dietary macronutrients in women with polycystic ovary syndrome. Int J Fertil Steril 2015;9:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourghassem Gargari B, Houjeghani S, Mahboob S, Farzadi L, Safaeian A.. Assessment of nutrients intake in polycystic ovary syndrome women compared to healthy subjects. Iran J Obstet Gynecol Infertil 2011;14:1–8. [Google Scholar]
- Prochaska JO, DiClemente CC.. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390–395. [DOI] [PubMed] [Google Scholar]
- Rajaeieh G, Marasi M, Shahshahan Z, Hassanbeigi F, Safavi SM.. The Relationship between intake of dairy products and polycystic ovary syndrome in women who referred to Isfahan University of Medical Science Clinics in 2013. Int J Prev Med 2014;5:687–694. [PMC free article] [PubMed] [Google Scholar]
- Rajaeieh G, Shokri Mashhadi N, Safavi M, Amini Pozveh Z, Pezeshki A.. The association between amino acid intake and polycystic ovary syndrome in women who referred to Isfahan University of Medical Science Clinics. Curr Res Nutr Food Sci 2018;5:11–17. [Google Scholar]
- Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, Mayo H, Singal AG.. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018;16:198–210.e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Chan SP, Spacey S, Anyaoku V, Johnston DG, Franks S.. thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol (Oxf) 1992;36:537–543. [DOI] [PubMed] [Google Scholar]
- Romualdi D, Immediata V, De Cicco S, Tagliaferri V, Lanzone A.. Neuroendocrine regulation of food intake in polycystic ovary syndrome. Reprod Sci 2018;25:644–653. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41–47. [DOI] [PubMed] [Google Scholar]
- Sam S, Ehrmann DA.. Pathogenesis and consequences of disordered sleep in PCOS. Clin Med Insights Reprod Health 2019;13:1179558119871269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl L, Bogensberger B, Hoffmann G.. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2018;118:74–100.e11. [DOI] [PubMed] [Google Scholar]
- Sedighi S, Amir Ali Akbari S, Afrakhteh M, Esteki T, Alavi Majd H, Mahmoodi Z.. Comparison of lifestyle in women with polycystic ovary syndrome and healthy women. Glob J Health Sci 2014;7:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahdadian F, Ghiasvand R, Abbasi B, Feizi A, Saneei P, Shahshahan Z.. Association between major dietary patterns and polycystic ovary syndrome: evidence from a case-control study. Appl Physiol Nutr Metab 2019;44:52–58. [DOI] [PubMed] [Google Scholar]
- Shahrokhi SA, Naeini AA.. The association between dietary antioxidants, oxidative stress markers, abdominal obesity and poly-cystic ovary syndrome: a case control study. J Obstet Gynaecol 2020;40:77–82. [DOI] [PubMed] [Google Scholar]
- Shishehgar F, Mirmiran P, Rahmati M, Tohidi M, Ramezani Tehrani F.. Does a restricted energy low glycemic index diet have a different effect on overweight women with or without polycystic ovary syndrome? BMC Endocr Disord 2019;19:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishehgar F, Tehrani FR, Mirmiran P, Hajian S, Baghestani AR, Moslehi N.. Factors influencing physical activity in women with polycystic ovary syndrome in comparison to eumenorrheic non hirsute women. Glob J Health Sci 2016b;8:56382. [DOI] [PubMed] [Google Scholar]
- Shishehgar F, Tehrani R, Mirmiran F, Hajian P, Baghestani S, Moslehi AR.. N. Comparison of dietary intake between polycystic ovary syndrome women and controls. Glob J Health Sci 2016a;8:54801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg A, Slinde F, Arvidsson D, Ellegård L, Gramatkovski E, Hallberg L, Hulthén L.. Energy intake in Swedish adolescents: validation of diet history with doubly labelled water. Eur J Clin Nutr 2003;57:1643–1652. [DOI] [PubMed] [Google Scholar]
- Soodi S, Keshavarz SA, Hosseini S, Abbasi B.. Dietary diversity score is inversely related to the risk of polycystic ovary syndrome in Tehranian women: a case-control study. Appl Physiol Nutr Metab 2021;1–6. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen RPM, Wiegel RE, Jansen PW, Laven JSE, KD S.. Polycystic ovary syndrome: a brain disorder characterized by eating problems originating during puberty and adolescence. Int J Mol Sci 2020;21:8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB.. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- Syngelaki AS, Campos M, Roberge S, Andrade W, Nicolaides KH.. Diet and exercise for preeclampsia prevention in overweight and obese pregnant women: systematic review and meta-analysis. J Matern Fetal Neonatal Med 2019;32:3495–3501. [DOI] [PubMed] [Google Scholar]
- Szczuko M, Szydłowska I, Nawrocka-Rutkowska J.. A properly balanced reduction diet and/or supplementation solve the problem with the deficiency of these vitamins soluble in water in patients with PCOS. Nutrients 2021;13:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay CT, Moran LJ, Harrison CL, Brown WJ, Joham AE.. Physical activity and sedentary behaviour in women with and without polycystic ovary syndrome: an Australian population-based cross-sectional study. Clin Endocrinol (Oxf) 2020;93:154–162. [DOI] [PubMed] [Google Scholar]
- Tay CT, Teede HJ, Boyle JA, Kulkarni J, Loxton D, Joham AE.. Perinatal mental health in women with polycystic ovary syndrome: a cross-sectional analysis of an Australian population-based cohort. JCM 2019a;8:2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay CT, Teede HJ, Hill B, Loxton D, Joham AE.. Increased prevalence of eating disorders, low self-esteem, and psychological distress in women with polycystic ovary syndrome: a community-based cohort study. Fertil Steril 2019b;112:353–361. [DOI] [PubMed] [Google Scholar]
- Teede H, Deeks A, Moran L.. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede HJ, Joham AE, Paul E, Moran LJ, Loxton D, Jolley D, Lombard C.. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity (Silver Spring) 2013;21:1526–1532. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, Andersen M, Azziz R. et al. ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602–1602.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thara C, Divakar S.. Assessment of nutritional status of PCOS women in Kerala. Int J Appl Home Sci 2017;4:717–722. [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Circulation 2002;106:3143–3421. [PubMed] [Google Scholar]
- Thompson FE, Kirkpatrick SI, Subar AF, Reedy J, Schap TE, Wilson MM, Krebs-Smith SM.. The National Cancer Institute’s dietary assessment primer: a resource for diet research. J Acad Nutr Diet 2015;115:1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FE, Subar AF, Loria CM, Reedy JL, Baranowski T.. Need for technological innovation in dietary assessment. J Am Diet Assoc 2010;110:48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson RL, Buckley JD, Moran LJ, Noakes M, Clifton PM, Norman RJ, Brinkworth GD.. Comparison of aerobic exercise capacity and muscle strength in overweight women with and without polycystic ovary syndrome. BJOG 2009;116:1242–1250. [DOI] [PubMed] [Google Scholar]
- Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB.. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015;3:968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y-H, Wang T-W, Wei H-J, Hsu C-Y, Ho H-J, Chen W-H, Young R, Liaw C-M, Chao JC-J.. Dietary intake, glucose metabolism and sex hormones in women with polycystic ovary syndrome (PCOS) compared with women with non-PCOS-related infertility. Br J Nutr 2013;109:2190–2198. [DOI] [PubMed] [Google Scholar]
- Turati F, Galeone C, Augustin LSA, La Vecchia C.. Glycemic index, glycemic load and cancer risk: an updated meta-analysis. Nutrients 2019;11:2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-McGrievy GM, Davidson CR, Wingard EE, Billings DL.. Low glycemic index vegan or low-calorie weight loss diets for women with polycystic ovary syndrome: a randomized controlled feasibility study. Nutr Res 2014;34:552–558. [DOI] [PubMed] [Google Scholar]
- van den Brink CM, Houben A, van Nierop P, Droomers M.. Validation of a Community Health Services Food Consumption Questionnaire in The Netherlands. Bilthoven, The Netherlands: RIVM National Institute for Public Health and the Environment, 2005. [Google Scholar]
- Vioque J, Navarrete-Muñoz EM, Gimenez-Monzó D, García-de-la-Hera M, Granado F, Young IS, Ramón R, Ballester F, Murcia M, Rebagliato M. et al. ; INMA-Valencia Cohort Study. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J 2013;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhai JX, Liu DW.. Serum folate, vitamin B12 levels and diabetic peripheral neuropathy in type 2 diabetes: a meta-analysis. Mol Cell Endocrinol 2017;443:72–79. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sun Y, Xu Q, Liu W, Wang P, Yao J, Zhao A, Chen Y, Wang W.. Higher dietary inflammation potential and certain dietary patterns are associated with polycystic ovary syndrome risk in China: a case-control study. Nutr Res 2022;100:1–18. [DOI] [PubMed] [Google Scholar]
- Wang Z, Groen H, Cantineau AEP, van Elten TM, Karsten MDA, van Oers AM, Mol BWJ, Roseboom TJ, Hoek A.. Dietary intake, eating behavior, physical activity, and quality of life in infertile women with PCOS and obesity compared with non-PCOS obese controls. Nutrients 2021a;13:3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Groen H, Cantineau AEP, van Elten TM, Karsten MDA, van Oers AM, Mol BWJ, Roseboom TJ, Hoek A.. Effectiveness of a 6-month lifestyle intervention on diet, physical activity, quality of life, and markers of cardiometabolic health in women with PCOS and obesity and non-PCOS obese controls: one size fits all? Nutrients 2021b;13:3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekker V, van Dammen L, Koning A, Heida KY, Painter RC, Limpens J, Laven JSE, Roeters van Lennep JE, Roseboom TJ, Hoek A.. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update 2020;26:942–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GA, Tugwell P, O’Connell D, Welch V, Peterson J, Shea B, Losos M.. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute; 2011. Oxford. asp; 2011.
- Wendel-Vos GC, Schuit AJ, Saris WH, Kromhout D.. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 2003;56:1163–1169. [DOI] [PubMed] [Google Scholar]
- Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA.. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 2010;95:2038–2049. [DOI] [PubMed] [Google Scholar]
- Wright C, Zborowski J, Talbott E, McHugh-Pemu K, Youk A.. Dietary intake, physical activity, and obesity in women with polycystic ovary syndrome. Int J Obes Relat Metab Disord 2004a;28:1026–1032. [DOI] [PubMed] [Google Scholar]
- Wright CE, Zborowski JV, Talbott EO, McHugh-Pemu K, Youk A.. Dietary intake, physical activity, and obesity in women with polycystic ovary syndrome. Int J Obes Relat Metab Disord 2004b;28:1026–1032. [DOI] [PubMed] [Google Scholar]
- Yin J, Hong X, Ma J, Bu Y, Liu R.. Serum trace elements in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2020;11:572384–572384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaeemzadeh N, Mottaghi A, Mohamadzadeh N, ziaiee S.. The study of dietary intake of macronutrients in four phenotypes of polycystic ovary syndrome based on Rotterdam criteria. RJMS 2018;25:46–56. [Google Scholar]
- Zaimzadeh N, Ziaie S, Mohammadzadeh N, Alizadeh Otaghvar H, Mottaghi A.. The study of dietary intake of micronutrients in four phenotypes of polycystic ovary syndrome separately based on Rotterdam criteria. RJMS 2018;25:59–68. [Google Scholar]
- Zawadski JK, Dunaif A.. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F (eds).Polycystic Ovary Syndrome. 1992. Boston, MA: Black-well Scientific Publications, 377–384. [Google Scholar]
- Zhang B, Zhai FY, Du SF, Popkin BM.. The China Health and Nutrition Survey, 1989-2011. Obes Rev 2014;15(Suppl 1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zhou W, Shi Y, Zhang J, Cui L, Chen ZJ.. Lifestyle and environmental contributions to ovulatory dysfunction in women of polycystic ovary syndrome. BMC Endocr Disord 2020;20:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Zeng L, Zhao J, Wu Q, Dong Y, Zou F, Gan L, Wei Y, Zhang W.. Association of magnesium intake with type 2 diabetes and total stroke: an updated systematic review and meta-analysis. BMJ Open 2020;10:e032240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JF, Li BX, Zhang Q.. Vitamin D improves levels of hormonal, oxidative stress and inflammatory parameters in polycystic ovary syndrome: a meta-analysis study. Ann Palliat Med 2021;10:169–183. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bo Y, Liu Y.. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: a dose-response meta-analysis of cohort studies. Lipids Health Dis 2019;18:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirak Sharkesh E, Keshavarz SA, Nazari L, Abbasi B.. The dietary inflammatory index is directly associated with polycystic ovary syndrome: A case-control study. Clin Endocrinol (Oxf) 2022;96:698–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding authors.