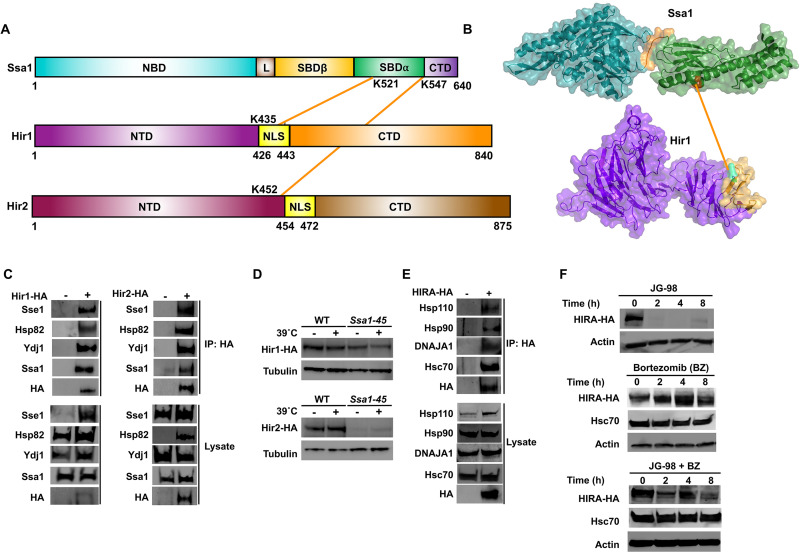
Fig 4. HIR complex is a novel client of Hsp70 in yeast and humans.
(A) Schematic representation of Ssa1-Hir1/Hir2 inter protein cross-links detected on SBD of Ssa1 and NLS of Hir1 and NTD of Hir2. (B) Ssa1-Hir1/2 cross-links mapped on the crystal structure of Ssa1, Hir1, and Hir2. (C) The Hir complex interacts with yeast chaperones. (D) Hir1 and Hir2 are dependent on Ssa1 for their stability. Indicated yeast cells were transformed with plasmids expressing HA-Hir1 or HA-Hir2 driven via the GAL1 promoter. Yeast were grown to mid-log in YPGalactose-URA media and then were either left untreated or were exposed to heat shock at 39°C for 90 min. Levels of Hir1 or Hir2 were assessed via western blot using antisera to indicated proteins. (E) HIRA interacts with chaperone complexes in mammalian cells. HEK293 cells were transfected with an HA-HIRA construct. After 24 h, total protein was extracted and HIRA complexes were purified via HA-magnetic beads. The purified HIRA complexes were analyzed by SDS-PAGE followed by western blotting using the indicated antisera. (F) Western blot analysis of HIRA upon addition of Hsp70 inhibitor JG-98 and proteasomal inhibitor bortezomib. CTD, C-terminal domain; HIR, histone regulator; NBD, nucleotide-binding domain; SBD, substrate-binding domain.