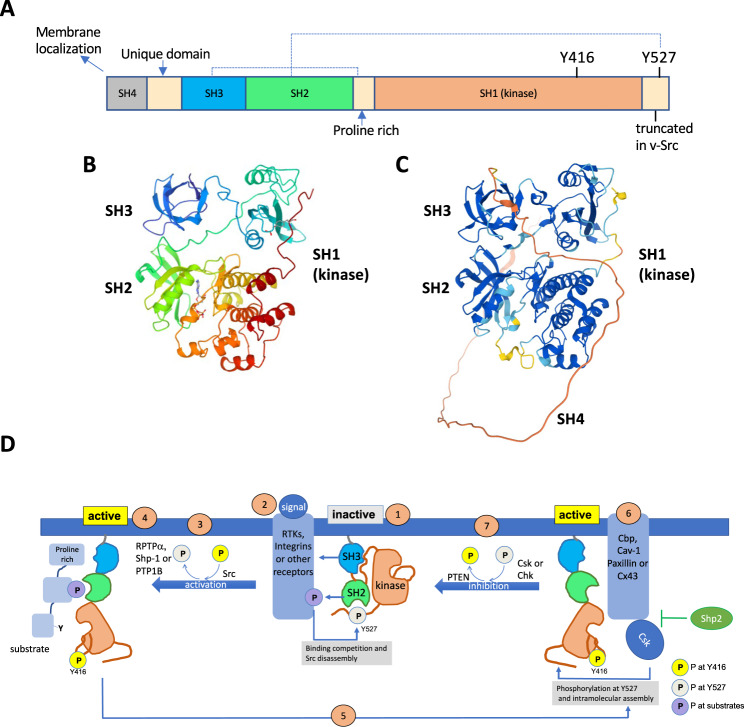
Fig. 1. Src structure and regulation.
A The domain structure of Src. Dashed lines depict intramolecular contacts established in the inactive conformation. B Three-dimensional structure of Src [145] (https://www.rcsb.org/structure/1FMK). C Src structure prediction with AlphaFold [144]. The comparison between these structures indicates the accuracy of AlphaFold prediction, which might be very useful for future studies of Src interactome, structure-function relationship and design of specific inhibitors. Note that B does not include the disordered SH4 region, which is shown in red in C. D Regulation of Src activity. (1) Src in the inactive, “closed” or assembled conformation with intramolecular contacts of the SH2 domain with phosphorylated Tyr527 and the SH3 domain with the proline rich region. (2) Src is activated in response to diverse signals; activated receptors compete for binding to Src SH2 or SH3 domains and disrupt Src intramolecular interactions. (3) Src is activated by autophosphorylation at Tyr416 and dephosphorylation at Tyr527, catalysed by the receptor protein tyrosine phosphatase α (PTPRA), the nonreceptor tyrosine phosphatase SHP-1 (PTPN6) or PTP1B. (4) Src in the active “open” or disassembled conformation with phosphorylated Tyr416. Src interacts with the SH3 and SH2 domain to the proline rich region and phosphotyrosine motif within the substrate for tyrosine phosphorylation. (5) Inhibition of Src requires the binding of active Src to CSK recruiting proteins (Cbp, Cav-1, Paxillin or Cx43). SHP-2 inhibits CSK recruitment. (6) CSK phosphorylates Src at Tyr527 promoting its intramolecular interaction with the SH2 domain. (7) Phosphatases, such as PTEN, remove phosphate at Tyr416 causing full Src inactivation (1).