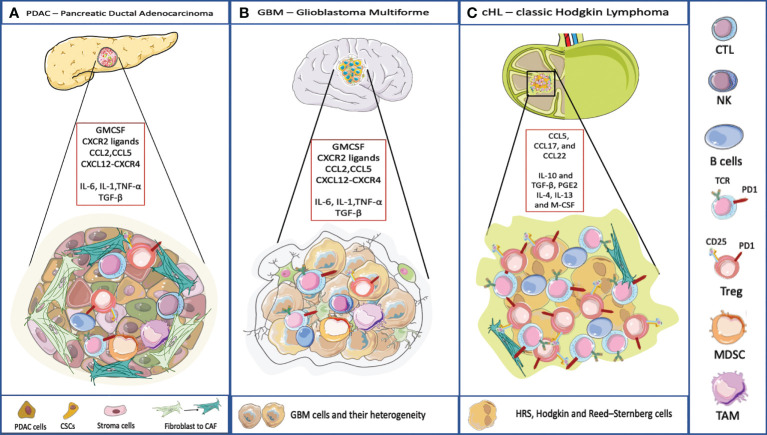
Figure 2.
TME and its composition in three different cancer model. (A) PDAC TME is characterized by a dense stroma, CAFs, and immune cells populations that crosstalk to the subpopulations of neoplastic cells that include cancer stem cells (CSCs). Pancreatic cancer cells typically express one or more mutated oncogenes or tumor suppressor genes (e.g. KRAS, TP53, CDKN2A, and SMAD4) that can interact with the TME, regulating stromal cells and the ECM in direct and indirect ways. The TME has highly diverse cellular and extracellular components. The CAFs are responsible for the secretion of extracellular matrix and soluble molecules which create a TME that favors tumor growth and resistance to the therapy. The TME is highly immunosuppressive, with the infiltration of TAMs, MDSC and Treg. The accumulation of CD4+ T cells and B cells contribute to carcinogenesis too. The soluble molecules released by PDAC cells or immune cells recreate an immunosuppressive microenvironment. IL-6, IL-1, TNF-α favor the polarization of macrophages into TAMs and they promote MDSC function, through the secretion of ECM. TGF-β is released by cancer cells and by immunosuppressive cells to favor a pro-tumoral environment. Here are represented some chemokines responsible for the recruitment of immune cells into the tumor, and involved in the redirection of the T cells, for example CCL5 attracts Tregs, CXCL12-CXCR4 restricts lymphocyte migration and keeps CTLs outside the tumor. (B) The TME surrounding malignant GBM is very complex. Indeed, the interaction between the immune system and the brain interior is problematic because of the presence of the blood-brain barrier. Even though, also in GBM the TME plays an important role. The GBM cells are heterogeneic they present antigen to CD4 and CD8 T cells that usually are dysfunctional and exhausted. Few B cells infiltrate in the brain. Moreover, GBM is characterized by an immunosuppressive and inflammatory microenvironment composed of cytokines (IL6, IL1-β, TGF-β, IL-10 and prostaglandin E2), chemokines, and regulatory immune-suppressive cells (Treg, TAMs, and MDSCs). Leukocyte recruitment to the tumor site is mediated by inflammatory chemokines from the CXC subfamily and CC group which attract leukocytes within tumor and exert pro- or anti-tumoral effects. CXCL8 is one of the inflammatory chemokines and the axis CXCL8-CXCR1/2 axis belongs to the most important and the best recognized regulatory factors in the development of CNS tumors. Moreover, CXCL8, CCL2, IL-6, contribute to MDSC generation (45). (C) cHL is characterized by a peculiar TME, most of the tumor is represented by dysfunctional T lymphocytes and by immunosuppressive cellular and acellular components. IL-10 and TGF-β, produced by RS, allow the accumulation of Tregs involved in the suppression of the activity of effector cells. IL-10 is a potent immune suppressive cytokine. Levels of IL-10 are elevated in up to a half of cHL patients and are correlated to disease aggressiveness and poor response to therapy. TGF-β production contributes to depress T-cell proliferation, cytokine release, and cytolytic activity. The soluble factor, prostaglandin E2 (PGE2), interferes with T cell receptor signaling, and decreases the cytotoxic response. IL-4, IL-13 and M-CSF attract myeloid cells and polarize macrophages into MDSC and TAMs (46).