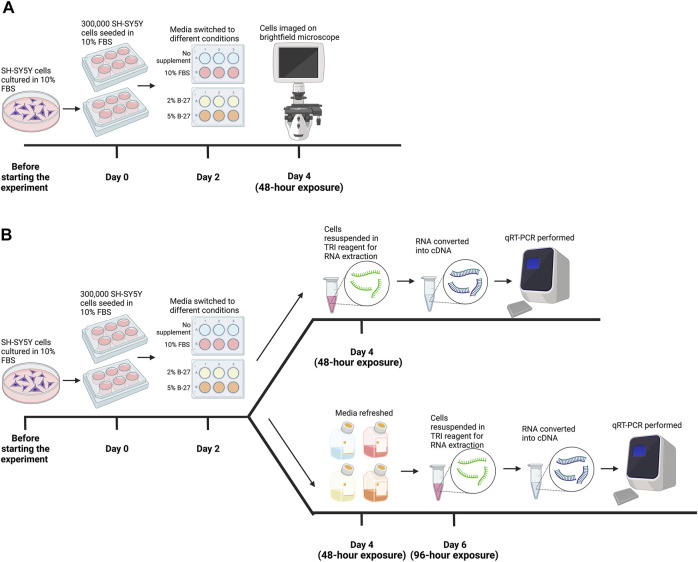
FIGURE 1.
Methodology for testing B-27 for SH-SY5Y cell culture. (A) Initial B-27 tests. Before the start of the experiment, SH-SY5Y cells were cultured in DMEM/F-12 + 10% FBS in 10-cm diameter Petri dishes until they reached approximately 70% confluency. Once cells reached 70% confluency (day 0), 300,000 SH-SY5Y cells per well were seeded into 6-well plates in DMEM/F-12 + 10% FBS. By day 2, cells reached approximately 70% confluency and media was switched to either: DMEM/F-12 only, DMEM/F-12 + 10% FBS, DMEM/F-12 + 2% B-27 and DMEM/F-12 + 5% B-27. After 48 h (day 4), cells were imaged on the EVOS FLoid microscope (Thermo Scientific). (B) SH-SY5Y cells were cultured in the various media conditions for 48 h as described in part (A). After 48 h (day 4), SH-SY5Y cells were either harvested in TRI reagent (Zymo Research) for RNA extraction, or the media was refreshed, and cells were cultured for a further 48 h. On day 6 (96-h exposure), SH-SY5Y cells were harvested in TRI reagent for RNA extraction. Following RNA extraction, RNA was converted into cDNA and qRT-PCR was performed on cells collected at both 48-h exposure and 96-h exposure time-points. DMEM/F-12, Dulbecco’s modified eagle medium/nutrient mixture F-12 with GlutaMAX supplement; FBS, fetal bovine serum; qRT-PCR, quantitative reverse transcription polymerase chain reaction. Figure made using BioRender.com.