FIGURE 3.
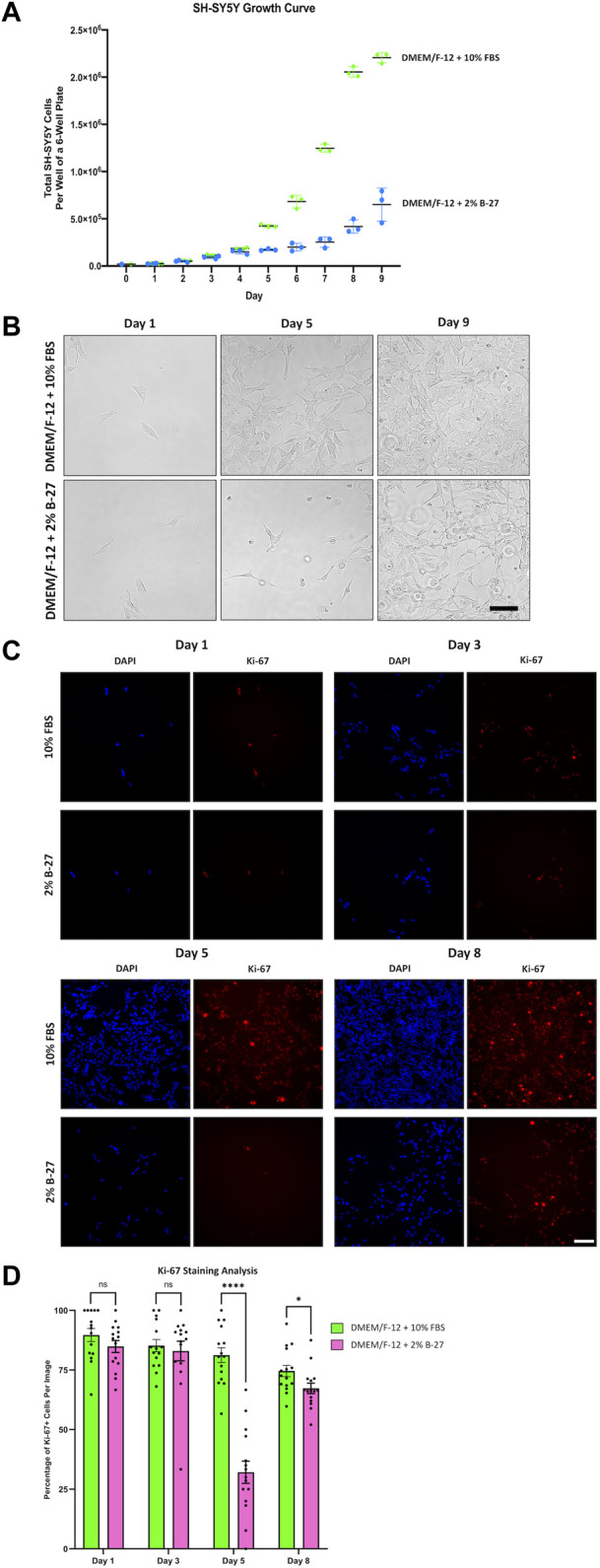
SH-SY5Y cells cultured in B-27 show reduced growth rate compared to SH-SY5Y cells cultured in FBS. (A) On Day 0, 20,000 SH-SY5Y cells per well were seeded into 6-well plates with DMEM/F-12 + 10% FBS. On Day 1, media was replaced with either DMEM/F-12 + 10% FBS or DMEM/F-12 + 2% B-27 supplement, and the number of cells per well was counted using a hemocytometer. Cell counts were performed at the same time daily for 9 days. (B) Representative brightfield mages were taken on Day 1, Day 5, and Day 9 using the EVOS FLoid microscope (Thermo Scientific). Scale bar = 100 μm. (C) On Day 0, 5,000 SH-SY5Y cells per well were seeded into 24-well plates with DMEM/F-12 + 10% FBS. On Day 1, media was replaced with either DMEM/F-12 + 10% FBS or DMEM/F-12 + 2% B-27 supplement. Cells were fixed on Day 1, Day 3, Day 5 and Day 8 with 4% PFA for 20 min. Proliferating cells were stained with 1:400 rabbit anti-Ki67 and visualized with 1:400 Alexa Fluor 555-conjugated donkey anti-rabbit (Invitrogen, #A31572) (red). Nuclei were counterstained with 1:5,000 DAPI (blue). Images taken at ×20 magnification through the DAPI (excitation 325–375 nm; emission 435–485 nm) and the TXR (excitation 540–580 nm; emission 592–668 nm) filter cubes on the Leica DMi8 Widefield microscope. Scale bar = 100 μm. (D) The number of Ki67+ cells and DAPI-stained nuclei were counted using the FIJI software, and the percentage of Ki-67+ cells per image calculated. Three independent replicates were performed, and 15 random images were analyzed per condition. Data presented as mean ± SEM. Statistical significance against the DMEM/F-12 + 10% FBS condition was determined using Unpaired t tests in GraphPad Prism version 9.0.0 for Mac, GraphPad Software, San Diego, California, United States, www.graphpad.com. ns = not significant; *p < 0.05; ****p < 1 × 10–4. DAPI: 4′,6-Diamidino-2-phenylindole; DMEM/F-12, Dulbecco’s modified eagle medium/nutrient mixture F-12 with GlutaMAX supplement; FBS, Fetal bovine serum; PFA, Paraformaldehyde; SEM, standard error of the mean.
