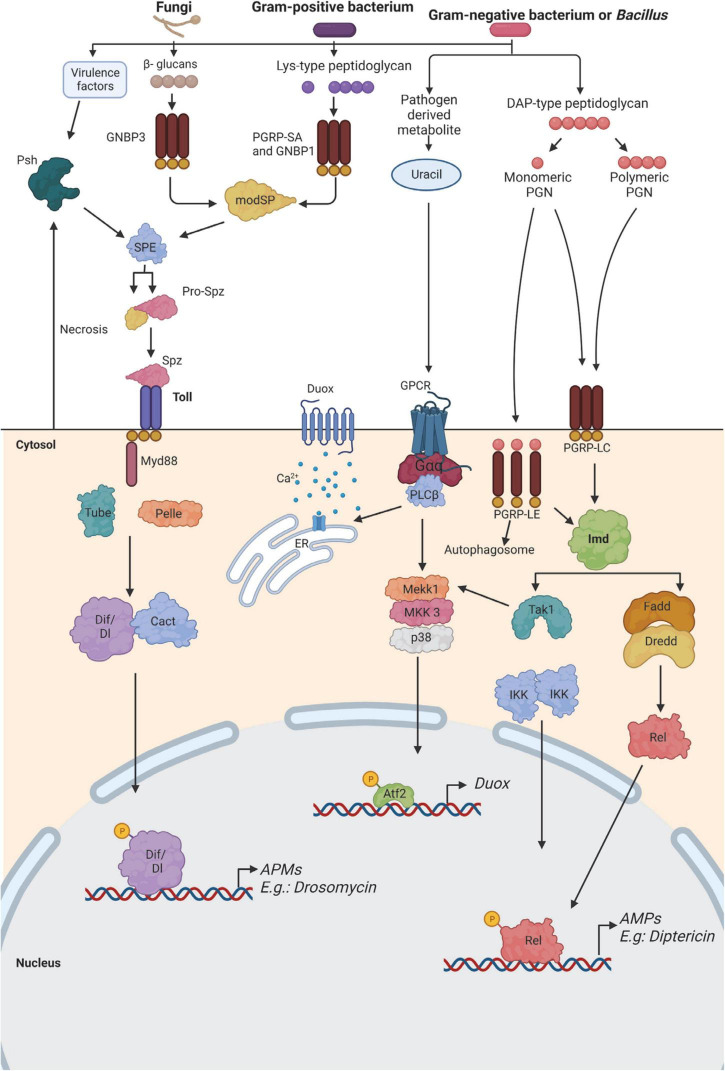
FIGURE 2.
Immune recognition of microorganisms in Drosophila. The two major pathways that sense bacteria and fungi in fruit flies are the Toll pathway (left) and the immune deficiency (Imd) pathway (right). Both pathways function in the fat body for the production of antimicrobial peptides (AMP) by activating the expression of NF-κB-like factors, which are highly conserved among species. In addition, the Imd pathway also functions in the epithelial surfaces. The Imd pathway is activated when DAP-type peptidoglycan from gram-negative bacteria, and some gram-positive, binds to Peptidoglycan Recognition Proteins (PGRPs), and this activation leads to the generation of AMP and synthesis of Duox enzyme for the production of reactive oxygen species (ROS). Gram-positive bacteria contain lys-type peptidoglycan, which is recognized by PGRP-SA and Gram Negative Bacteria Protein (GNBP) 1, and GNBP3 binds to β-glucans of yeasts and fungi, leading to the activation of the Toll pathway. This pathway can also be triggered by danger signals like proteases or abnormal cell death that activates the protease Persephone (Psh). In all cases, the activation of the Toll pathway triggers a proteolytic cascade that activates the protease Spätzle- processing enzyme (SPE). This protein will cleave Spätzle (Spz). As a result of the activation of the Toll pathway, the transcription factors Dorsal-related immunity factor (Dif) or Dorsal (Dl) will translocate to the nucleus, thus inducing the expression of AMP genes like drosomycin. Similarly, the activation of the Imd pathway induces the nuclear translocation of the transcription factor Relish (Rel) and induction of the expression of AMP genes, such as diptericin. The generation of ROS is induced by the activation of Duox in the presence of uracil. This is caused by the activation of a G protein-coupled receptor (GPCR), which promotes the release of calcium from the endoplasmic reticulum. This signaling pathway, together with the activation of the Imd pathway, contributes to the expression of the Duox enzyme during infection. Atf2, activating transcription factor 2; Dredd, death-related ced-3/Nedd2-like caspase; Fadd, FAS-associated death domain ortholog; Gαq, G protein αq-subunit; IKK, inhibitor of NF-κB kinase; MKK3, MAPK kinase 3; modSP, modular serine protease; Tak1, TGFβ-activated kinase 1. Adapted from Buchon et al. (2014) and Younes et al. (2020).