Abstract

This study facilitates the synthesis of a graphitic carbon nitride/cesium tungsten oxide (g-C3N4@Cs0.33WO3) heterojunction using a solvothermal method. The photocatalytic activities of the prepared samples were examined for the photodegradation of colorless antibiotics, namely tetracycline, enrofloxacin, and ciprofloxacin, as well as cationic and anionic dyes, such as methyl orange, rhodamine B, neutral red, and methylene blue, under full-spectrum solar light. We have purposely selected different kinds of wastewater pollutants of colorless antibiotics and cationic and anionic organic dyes to investigate the potential application of this heterojunction toward different groups of water pollutants. The results revealed that the g-C3N4@Cs0.33WO3 heterojunction showed an outstanding photocatalytic activity toward all the pollutants with concentrations of 20 ppm each at pH 3 by photocatalytically removing 97% of tetracycline within 3 h, 98% of enrofloxacin within 2 h, 97% of ciprofloxacin within 2.25 h, 98% of methylene blue in 1 h, 99% of rhodamine B within 2 h, 99% of neutral red in 1.25 h, and 95% of methyl orange in 2 h. These findings indicate that the developed photocatalyst possesses excellent photocatalytic properties toward seven different water pollutants that make it a universal photocatalyst. The developed g-C3N4@Cs0.33WO3 oxide heterojunction also presented a photocatalytic performance better than those of reported solar light active photocatalysts for photodegradation of rhodamine B and tetracycline. The efficient photocatalytic performance of the heterojunction can be ascribed to its extended light-absorbing ability, effective charge separation and fast charge transfer properties, and a high surface area. Moreover, an active species detection experiment also confirmed that superoxide radicals, hydroxyl radicals, and holes played significant roles in the photocatalysis of the organic dyes and tetracycline.
1. Introduction
Currently, environmental pollution and energy shortages have become major issues worldwide and immense efforts are being made to address them.1 Among the several types of environmental pollution, water pollution caused by antibiotics and organic dyes is a major problem that requires immediate action. Tetracycline (TC) is one among the various types of antibiotics used in daily life to prevent and treat microbial infections.2 It is widely utilized because of its low cost and the broad antibacterial spectrum. It is nonbiodegradable and cannot be absorbed by the animal intestine, and 90% of it is excreted into the environment, which causes environmental pollution and affects the health of living organisms.3 Therefore, the development of an effective and efficient method for its removal is very crucial. Other types of water pollutants are organic dye effluents that are released from different industries and have low biodegradability, high levels of chemical oxygen demand, and harmful effects on human health and the environment.4,5 Therefore, their presence is a global challenge to society and needs to be eliminated to achieve a conducive and safe environment. Thus, the development of an effective, simple, low-cost, and efficient method for removing these pollutants is essential. Previously, different methods, including adsorption, Fenton oxidation, biological filtration, chemical precipitation, photocatalysis, and ozone oxidation, have been developed for wastewater treatments.6−10 Among these methods, photocatalysis has received significant attention11 due to its simple operation strategy and high efficiency as well as low cost, power consumption, and eco-friendliness.12,13 Therefore, the development of suitable and effective photocatalysts is required for environmental remediation.14
Solar energy is an economical, abundant, clean, renewable, and green source of energy. The sun provides a continuous and intense light energy to the surface of the earth and is the most promising renewable source of energy for environmental treatments.15 The total solar energy comprises ultraviolet (UV), visible (Vis), and near-infrared (NIR) light, which constitutes 5%, 46%, and 49%, respectively.16−18 However, most conventional photocatalysts, such as TiO2, ZnO, Ag3VO4, MoS2, BiS2, TiO2@graphene, Pt/Bi@TiO2, GO@ZnO, D-g-C3N4@Bi5O7I, TiO2@SiO2@Fe3O4,19−22 and Fe3O4, are only active in UV and Vis light or in a combination of the two, which constitutes only 5% or 50% of the solar light. This leaves the NIR light unutilized, which constitutes 50% of the solar spectrum. Therefore, the development of solar light active photocatalysts that can absorb full-spectrum solar light from NIR–Vis–UV for environmental remediation is crucial.23
Previous studies have found that the formation of heterostructures from different materials that absorb in the different regions of the solar light spectrum can improve the utilization of solar energy and enhance the photocatalytic activity for wastewater treatments. Therefore, the design and selection of suitable materials to form heterostructures that can absorb full-spectrum solar light are necessary. Interestingly, tungsten bronze based materials MxWO3 (0 < x < 1, M = Cs, Rb, K, Na, Li) are suitable candidates for this purpose due to their excellent NIR and UV light absorption properties.7,24 In particular, Cs0.33WO3 has attracted attention in various fields, such as water evaporation,25 photocatalysis,26 gas sensors,27 smart window coatings, and photothermal ablation cancer treatments,25,28 due to its outstanding NIR light absorption property. The mixed-valence W5+ and W6+ as well as large oxygen vacancies make cesium tungsten oxide a suitable material for photocatalysis. Nevertheless, Cs0.33WO3 suffers from Vis light absorption, which hinders its use as a solar light photocatalyst.25 Thus, it is mandatory to select materials that have excellent Vis light absorption properties to develop photocatalyst heterostructures to enable the efficient utilization of full-spectrum solar light.16
Recently, graphitic carbon nitride (g-C3N4), which is a metal-free semiconductor, has received considerable attention in the field of photocatalysis owing to its eco-friendliness, simple preparation method, biocompatibility, high stability, moderate band gap, low cost, and excellent Vis light absorption.29 Due to these merits, it is being applied for the photodegradation of organic pollutants, CO2 reduction,30 and water splitting in environmental remediation. However, it suffers from the inefficient utilization of the full-spectrum solar light, small specific surface area, easy aggregation of nanosheets, and fast recombination of photogenerated charge carriers that hinder its use in practical applications.31 Various methods have been developed to overcome these drawbacks, among which heterojunction formation with suitable materials is a promising strategy to improve the photocatalytic activity.32 For instance, different types of heterojunctions such as but not limited to g-C3N4@WO3,33 UiO-66@g-C3N4,34 Co3O4@g-C3N4,35 and red phosphor@g-C3N4 have been developed and presented excellent photocatalytic performance compared to their respective bare materials. Different Cs0.33WO3-based heterostructure photocatalysts such as CdS@Cs0.33WO3,36 SiO2@Cs0.33WO3,37 ZnO@Cs0.33WO3,38 and AgBr@Cs0.33WO339 have also been developed and their findings reveal that the heterostructure showed outstanding photocatalytic properties compared with the two pristine materials. Previous works have been reported where Cs0.33WO316,24 was utilized as a UV- and NIR-absorbing photocatalyst. By taking advantage of the excellent UV and NIR absorption property of Cs0.33WO3, we have developed a full-spectrum solar light active g-C3N4@Cs0.33WO3 heterojunction photocatalyst for antibiotic degradation. Herein, g-C3N4 was chosen due to its good visible light absorbing property, eco-friendliness,40 suitable band-gap energy,41 lack of metal, and good stability.42 Previously, Gu et al. developed a g-C3N4@Cs0.33WO3 heterojunction and it exhibited excellent photocatalytic activity for CO2 reduction,26 which shows its potential applicability in environmental remediation. However, we report for the first time the photocatalytic activity of the g-C3N4@Cs0.33WO3 heterojunction on the photodegradation of colored and colorless pollutants under solar light irradiation.
The limited full-spectrum solar light absorption properties of most of the developed photocatalysts are the main drawbacks in the utilization of all the irradiated solar light region for environmental remediation. In this study, a full-spectrum solar light active g-C3N4@CsCs0.33WO3 heterojunction was developed via a solvothermal method by taking into account the excellent visible light absorbing property of g-C3N4 and outstanding UV as well as NIR optical properties of Cs0.33WO3. The photocatalytic properties of the fabricated heterojunction have been demonstrated toward the photodegradation of colorless antibiotics, namely tetracycline (TC), ciprofloxacin, and enrofloxacin, as well as cationic and anionic dyes. Moreover, the effect of experimental parameters such as pH, concentration of pollutant, and catalyst dosage on the photocatalytic performance of the developed photocatalyst was investigated. The photodegradation of intermediate products was also systematically studied. Based on the experimental findings, a possible photocatalytic mechanism for the photocatalytic performance of the fabricated g-C3N4@Cs0.33WO3 heterojunction is proposed.
2. Materials and Methods
2.1. Materials
Tungsten(VI) chloride (99.9%, Acros, New Jersey, USA), Melamine (99%. Poland), cesium hydroxide (99.9%, Alfa Aesar, Wardhill, MA, USA), anhydrous ethanol (99.5%, ECOs), glacial acetic acid (AENCORE, Surrey Hills, Australia), rhodamine B (98+%), methylene blue (99%), methyl orange (99%), tetracycline (98), enrofloxacin (99+%), and ciprofloxacin (98+%) (Sigma-Aldrich, New Jersey, USA) were purchased from the suppliers indicated.
2.2. Synthesis of g-C3N4@Cs0.33WO3 Heterojunction
First, g-C3N4 was synthesized from melamine according to the method described in our previously published study.25 Thereafter, the desired amount of g-C3N4 was dissolved in 80 mL of ethanol and stirred for 1 h. Subsequently, 0.595 g of WCl6 was added to the above solution, and the mixture was stirred until a homogeneous solution was obtained. Afterward, 0.13 g of CsOH·H2O was introduced and the mixture was stirred further for 10 min. Then, 20 mL of glacial acetic acid was added and a solvothermal reaction was allowed to occur for 20 h at 240 °C in an autoclave. After the reaction was completed, the autoclave was cooled to room temperature naturally, and the obtained product was washed four times with ethanol and dried in an oven at 60 °C for 8 h.24
2.3. Characterizations
The optical properties of the samples were investigated using UV–Vis–NIR spectrophotometry (JASCO V 670, Tokyo, Japan). The morphologies of the samples were studied by field-emission scanning electron microscopy (FESEM, JSM 6500F, JEOL Tokyo, Japan), and an N2 adsorption measurement was done at 77 K to determine the specific surface area of the sample using a Quantachrome iQ-MP gas adsorption analyzer (BEL JAPAN, INC.). The concentrations of the pollutants were studied using UV–Vis–NIR spectrophotometry in the wavelength range of 200–800 nm. The photocurrent response tests and electrochemical impedance spectroscopy (EIS) were performed using a 5000 electrochemical workstation (Technology Corp., Taipei, Taiwan). The total organic carbon was investigated with a Vario TOC select Elemenetar instrument, and the intermediate products were studied by a gas chromatograph–mass spectrometer detector (5977B GC/MSD and 7890B GC) system.
2.4. Study of Photocatalytic Properties
The photocatalytic activities of the as-prepared photocatalysts were evaluated for the photodegradation of pollutants, such as TC, enrofloxacin, ciprofloxacin, methylene blue (MB), rhodamine B (Rh B), red dye, and methyl orange (MO) under full solar light illumination. Typically, 20 mg of the photocatalyst was dispersed in 100 mL of a solution containing 20 ppm of each pollutant. Before photocatalysis, the mixture was stirred in the dark for 30 min to attain an adsorption–desorption equilibrium. Then, a Xe lamp (500 W Prosper Optoelectronic, Co., Ltd., Taipei, Taiwan) with a wavelength range of 250–2500 nm was used as the light source. The distance between the lamp and photocatalysis reactor was 35 cm, and the optical intensity of solar light was 0.25 W/cm2. The light was irradiated for 2 h in the case of MO, Rh B, and enrofloxacin, for 1 h in the case of MB, and for 3, 2.25, and 1.25 h for TC, ciprofloxacin, and NR, respectively. A 4 mL portion of the solution was taken every 15 min and centrifuged. The concentration of each pollutant left in the supernatant solution was analyzed using a UV–vis–NIR spectrometer (V-670, JASCO, Tokyo Japan).43−45 Finally, the degradation efficiencies of organic dyes and TC were calculated based on eq 1(46−51)
| 1 |
where Ct is the concentration at irradiation time t and C0 is the initial concentration of the pollutant.
2.5. Photoelectrochemical Measurements
EIS was used in a conventional three-electrode configuration. The as-prepared samples were cast on 1 × 1 cm2 titanium, which served as the working electrode, and a Ag/AgCl electrode, platinum electrode, and 0.1 M KCl solution were used as the reference electrode, counter electrode, and electrolyte, respectively. Benzoquinone (BQ), isopropanol (IPA), and ethylenediaminetetraacetic acid (EDTA) were used as superoxide radical, hydroxyl radical, and hole scavengers, respectively, in the active species study.
3. Results and Discussion
3.1. Characterization of the g-C3N4@Cs0.33WO3 Heterojunction
Figure 1a displays the FESEM image of the g-C3N4@Cs0.33WO3 heterojunction, which demonstrates that the g-C3N4 nanoparticle (shown in red) is adhered to the surface of the Cs0.33WO3 nanorods (encircled in yellow), indicating a good contact between g-C3N4 and Cs0.33WO3. The energy-dispersive X-ray spectrum (EDS) presented in Figure 1b also confirms the existence of Cs, W, O, N, and C in the g-C3N4@Cs0.33WO3 heterojunction, which shows the coexistence of Cs0.33WO3 and g-C3N4. The crystal structures of the fabricated g-C3N4, Cs0.33WO3, and g-C3N4@Cs0.33WO3 were investigated and reported in our previous study.25 The actual atomic and mass percentages of elements in the developed Cs0.33WO3@g-C3N4 heterojunction (Figure 1b inset) were also almost the same as the theoretical mass and atomic percentages of each element used in the synthess, that confirms the successful fabrication of the heterojunction.
Figure 1.
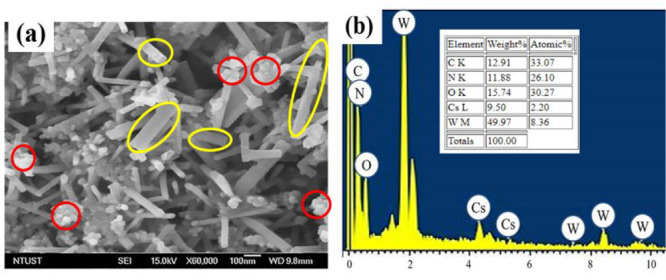
(a) FESEM image and (b) corresponding EDS spectrum of the g-C3N4@Cs0.33WO3 heterojunction.
3.2. Photoelectrochemical and Optical Analyses
Figure 2a shows the EIS results of g-C3N4, Cs0.33WO3, and the g-C3N4@Cs0.33WO3 heterojunction, and the figure depicts that the semicircle radius of the g-C3N4@Cs0.33WO3 heterojunction is much smaller than those of g-C3N4 and Cs0.33WO3, indicating the excellent charge transfer of the heterojunction. The photoluminescence (PL) spectra (Figure 2b) indicated that the g-C3N4@Cs0.33WO3 heterojunction presented the lowest PL peak intensity followed by Cs0.33WO3, which shows the suppression of photogenerated electron–hole recombination and efficient charge separation, which is crucial for achieving an effective photocatalysis. In contrast, g-C3N4 exhibited strong PL peaks, indicating a high recombination rate of hole–electron pairs in g-C3N4, which is not ideal for an effective photocatalysis. The photocurrent response results displayed in Figure 2c also indicate that the g-C3N4@Cs0.33WO3 heterojunctions exhibit strong photocurrent responses compared to those of the bare materials, which indicates that the heterojunction possesses an excellent light absorption and conversion efficiency. The enhancement of the photocurrent response shows that the heterojunction can efficiently generate and transfer photogenerated charge carriers, which causes efficient electron–hole pair separation and a high photocatalytic performance.6 The optical properties of the synthesized samples were also investigated in full-spectrum solar light (UV–vis–NIR region), and the results (Figure 2d) show that g-C3N4 exhibits a strong optical absorption in the Vis region but limited optical absorption in the UV and NIR regions. Cs0.33WO3 exhibited significant NIR and UV light absorption properties, which are crucial for full-spectrum solar light absorption. Consequently, the developed g-C3N4@Cs0.33WO3 heterojunction demonstrated Vis, UV, and NIR light absorption capabilities throughout the solar spectrum, which is very important for improving the photocatalytic activity.
Figure 2.

(a) EIS, (b) photocurrent response, (c) PL spectra, and (d) DRS spectra of Cs0.33WO3, g-C3N4, and the g-C3N4@Cs0.33WO3 heterojunction.
Moreover, the band gap of the photocatalyst, which is an important parameter in photocatalysis, was determined by using the Kubelka–Munk formula52 from DRS, and the plots of (αhν)1/2 versus hν, where h, α, and ν denote Planck’s constant, absorption coefficient, and light frequency, respectively, are displayed in Figure 3a–c. From the results, the calculated band gap energies (Eg) of g-C3N4, Cs0.33WO3, and g-C3N4@Cs0.33WO3 were 2.50, 2.58, and 2.26 eV, respectively. The flat-band potentials of g-C3N4 and Cs0.33WO3 were determined by the Mott–Schottky method, and g-C3N4 (Figure 3d) as well as Cs0.33WO3 (Figure 3e) demonstrated positive slopes, which indicated that both materials are n-type semiconductors.46 As can be seen from the linear extrapolation of the results to the x intercepts of Figure 3, the flat band potentials versus an Ag/AgCl electrode for Cs0.33WO3, and g-C3N4 are −0.75, and −1.0 V, respectively.53 By using the formula for the normal hydrogen electrode ENHE = EAg/AgCl + 0.197 V,54,55 the corresponding values vs NHE of Cs0.33WO3, and g-C3N4 are −0.55, and −0.8 V, respectively. The conduction band (CB) potentials of n-type semiconductors are almost equal to the flat-band potentials. Therefore, the corresponding CBs of Cs0.33WO3 and g-C3N4 are −0.55 and −0.8 eV, respectively, as calculated according to the formula EVB = Eg + ECB,56 in which the determined valence bands (VBs) of Cs0.33WO3 and g-C3N4 are 2.02 and 1.7 eV versus the normal hydrogen electrode (NHE), respectively. The Eg values of g-C3N4 and Cs0.33WO3 are 2.50 and 2.58 eV, respectively, as presented in Figure 3a,b.
Figure 3.

(a–c) Band gaps for g-C3N4, Cs0.33WO3, and g-C3N4@ Cs0.33WO3 respectively. (d, e) Mott–Schottky plots of g-C3N4 and Cs0.33WO3, respectively.
Moreover, the specific surface areas and pore size N2 adsorption–desorption isotherms of the fabricated samples were investigated, and the results given in Figure 4 show that all of the photocatalysts presented type IV hysteresis loops according to the Brunauer–Deming–Deming–Teller classification.27 The calculated specific surface area, pore diameter, and pore volume are also displayed in Table 1, and the results show that the synthesized g-C3N4@Cs0.33WO3 heterojunction presented the highest specific surface area of 51.74 m2/g as compared to those of the bare materials Cs0.33WO3 and g-C3N4. This improved surface area provides a large active site for the adsorption of pollutants on the photocatalyst, further enhancing the photocatalytic activity of the heterojunctions.57
Figure 4.

N2 adsorption–desorption isotherms of Cs0.33WO3, g-C3N4@Cs0.33WO3, and g-C3N4.
Table 1. BET Specific Surface Areas, Pore Diameters, and Pore Volumes of Photocatalysts.
| photocatalyst | specific BET surface area (m2/g) | pore diameter (nm) | pore volume (cm3/g) |
|---|---|---|---|
| g-C3N4 | 37.29 | 28.15 | 0.26 |
| Cs0.33WO3 | 36.64 | 36.49 | 0.33 |
| g-C3N4@Cs0.33WO3 | 51.74 | 23.84 | 0.30 |
3.3. Study of Photocatalytic Activity on Organic Dyes
The photocatalytic activities of the as-synthesized photocatalysts were investigated for different types of pollutants composed of cationic and anionic organic dyes. First, the photocatalytic properties of the fabricated photocatalysts toward the anionic dye MO were investigated, and the results shown in Figure 5a indicate that the g-C3N4@Cs0.33WO3 heterojunction exhibited excellent photocatalytic activity by degrading 95.4% within 2 h under solar light irradiation. Cs0.33WO3 and g-C3N4 degraded only 41.4% and 73.7% of MO, respectively, under the same conditions. Moreover, the universal applicability of the developed photocatalysts was evaluated by applying them to different cationic dyes and Figure 5b depicts that the g-C3N4@Cs0.33WO3 heterojunction showed excellent photocatalytic activity toward MB by degrading 98.2% of it in 1 h, whereas the photocatalytic activities of bare g-C3N4 and Cs0.33WO3 were 63.2% and 38.4%, respectively, under the same experimental conditions. Similarly, the synthesized heterojunction displayed significant photocatalytic properties toward Rh B by decomposing 99.2% of Rh B within 2 h (Figure 5c), while only 17.4% and 73.5% of Rh B were degraded by Cs0.33WO3 and g-C3N4, respectively. Figure 5d also shows that the g-C3N4@Cs0.33WO3 heterojunction possesses excellent photocatalytic activity toward the neutral red (NR) dye, and 99.3% can be decomposed in 75 min, whereas only 57.4% and 54.1% could be decomposed by g-C3N4 and Cs0.33WO3 under similar conditions, respectively. The excellent photocatalytic activity of the g-C3N4@Cs0.33WO3 heterojunction toward all pollutants compared to those of the bare g-C3N4 and Cs0.33WO3 can be ascribed to the extension of the optical absorption region, high surface area, and effective charge separation.6 In addition, the photocatalytic performance of the developed g-C3N4@Cs0.33WO3 heterojunction was also compared with those of previously reported solar light active photocatalysts for the photodegradation of Rh B. The findings in Table 2 show that the developed g-C3N4@Cs0.33WO3 heterojunction exhibited an excellent photocatalytic performance compared to those reported in the literature. This indicates that the fabricated photocatalyst is a promising candidate for application in environmental remediation for the treatment of organic dyes and can be employed for real-life applications.
Figure 5.

Photocatalytic degradation efficiency of the as-prepared samples on (a) MO, (b) MB, (c) Rh B, and (d) NR, under solar light irradiation.
Table 2. Comparison of the Photocatalytic Activity of the Developed g-C3N4@Cs0.33WO3 Heterojunction with Those of the Previously Reported Solar Light Photocatalysts for the Photodegradation of Rh B Dye.
The rate of the reaction kinetics of the photocatalyst was also studied by fitting the experimental results with a pseudo-first-order reaction model according to eq 2(7)
| 2 |
where k is the kinetic rate constant, t is the irradiation time, and C0 and Ct are the concentrations of pollutants at time 0 and time t, respectively. Figure S1a–d shows the linear plots of ln(Ct/C0) versus the irradiation time for MO, MB, Rh B, and red dye. The reaction rate constant (k) was calculated and is provided in Table 3. The reaction rate of the g-C3N4@Cs0.33WO3 heterojunction is faster than those of bare g-C3N4 and Cs0.33WO3 for all of the studied pollutants (MO, MB, Rh B, and NR). For example, the reaction rate kinetic constant of the heterojunction is about 1.8 times faster than that of g-C3N4 and is about 12 times faster than that of Cs0.33WO3 for the photodegradation of Rh B dye under the same experimental conditions. The improved reaction rate of the g-C3N4@Cs0.33WO3 heterojunction could be due to the large surface area, extended full-spectrum light absorbance, narrowing of the band gap, and effective charge transfer of photogenerated charge carriers.57
Table 3. Pseudo-First-Order Rate Constants (min–1) for MO, MB, Rh B, and NR Dye Degradation under Solar Light Irradiation.
|
k (10–3 min–1) |
||||
|---|---|---|---|---|
| photocatalyst | MO | Rh B | MB | NR |
| g-C3N4 | 9.7 | 10.8 | 16.4 | 12.5 |
| Cs0.33WO3 | 4.4 | 1.6 | 7.8 | 10.4 |
| g-C3N4@Cs0.33WO3 | 19.6 | 19.5 | 40.3 | 31.5 |
To evaluate the light utilization efficiency of the developed g-C3N4@Cs0.33WO3 heterojunction, the apparent quantum efficiency (Φ) of the photocatalyst was calculated according to eq 3(62)
| 3 |
where v is the volume of the reaction solution (L), NA is Avogadro’s number (6.022 × 1023 molecules/mol), CA is the concentration of the reactant A (mol/L), Nph is the number of photons supplied in time t, and t is reaction time. Herein, the reactant is MB.
The calculated apparent quantum efficiencies under incident light of 3 × 1015 photons/s were 2.5%, 0.85%, and 0.53% for the g-C3N4@Cs0.33WO3 nanocomposite, g-C3N4, and Cs0.33WO3 photocatalysts, respectively. This finding depicts that the g-C3N4@Cs0.33WO3 heterojunction had apparent quantum efficiency of about 3 times that of g-C3N4 and almost 5 times that of Cs0.33WO3. The heterojunction formation has largely improved the apparent quantum efficiency, which improved the photocatalytic performance of the developed material.
The total organic carbon (TOC) was investigated to evaluate the mineralization property of the g-C3N4@Cs0.33WO3 heterojunction on Rh B and TC pollutants under solar light irradiation. The finding shown in the Figure 6 presents the TOC of the Rh B before and after photocatalysis by the g-C3N4@Cs0.33WO3 heterojunction. The decrease of the TOC in the Rh B of 99.4% after photocatalysis confirms the mineralization of the Rh B into CO2 and H2O.63,64 Similarly, the concentrations of TOC before and after photodegradation of TC were also determined, and the finding reveals that 99% of TOC was removed during photodegradation of TC, which confirms the excellent mineralization properties of the developed photocatalyst during photodegradation of TC.65
Figure 6.

TOC analysis of Rh B and TC pollutants before and after their photodegradation.
3.4. Photocatalytic Activity on Antibiotics
The photocatalytic activity of the heterojunction as well as bare samples was evaluated by photodegradation of antibiotics such as enrofloxacin, ciprofloxacin, and tetracycline under simulated solar light. The result presented in Figure 7a indicates that the g-C3N4@Cs0.33WO3 heterojunction exhibited excellent photocatalytic activity toward ciprofloxacin by degrading 98% of it within 135 min under solar light irradiation. However, Cs0.33WO3 and g-C3N4 degraded only 56% and 64.2% of ciprofloxacin, respectively, under the same experimental conditions. Moreover, the as-prepared g-C3N4@Cs0.33WO3 heterojunction also displayed outstanding photocatalytic properties toward enrofloxacin by decomposing 97.1% of it within 2 h (Figure 7b), while only 68% and 73.5% of enrofloxacin were degraded by Cs0.33WO3 and g-C3N4, respectively. Furthermore, the g-C3N4@Cs0.33WO3 heterojunction also exhibited significant photocatalytic activity toward TC by decomposing 97% of it within 3 h (Figure 7c), whereas 83.7% and 61.8% degradations of TC were achieved by g-C3N4 and Cs0.33WO3, respectively. Furthermore, the photocatalytic performance of the developed g-C3N4@Cs0.33WO3 heterojunction was compared with those of previously reported photocatalysts for the photodegradation of TC, and the results are displayed in Table 4. The finding displays that the developed g-C3N4@Cs0.33WO3 heterojunction shows better TC photodegradation than those reported photocatalysts.
Figure 7.

Photocatalytic degradation efficiency of the as-prepared samples on (a) ciprofloxacin (b) enrofloxacin, and (c) TC, under solar light irradiation.
Table 4. Comparison of the Photocatalytic Performance of the Fabricated g-C3N4@Cs0.33WO3 Heterojunction with Those of the Previously Reported Solar Light Photocatalysts for Photodegradation of the Colorless Antibiotic TC.
| photocatalyst | pollutant | irradiation time (min) | light source | photodegradation efficiency (%) | ref |
|---|---|---|---|---|---|
| TiO2 graphene aerogel | TC | 180 | solar | 43 | (61) |
| Bi2WO6 /TiO2 | TC | 180 | solar | 92 | (66) |
| Co-doped UiO-66 | TC | 180 | solar | 94 | (67) |
| Bi2MoO6/WO3 | TC | 180 | solar | 86 | (68) |
| FeNi3/SiO2/CuS | TC | 200 | solar | 81 | (69) |
| g-C3N4@Cs0.33WO3 | TC | 180 | solar | 97 | this work |
Figure S2a–c shows the linear plots of ln(Ct/C0) versus the irradiation time for enrofloxacin, ciprofloxacin, and TC photodegradations. The reaction rate constants (k) were calculated and are provided in Table 5. The reaction rate of the g-C3N4@Cs0.33WO3 heterojunction is faster than that of bare g-C3N4 and Cs0.33WO3 for all of the studied pollutants (enrofloxacin, ciprofloxacin, and TC). As can be seen from Table 5, the reaction rate kinetic constant for the g-C3N4@Cs0.33WO3 heterojunction is greater than those of bare g-C3N4 and Cs0.33WO3 for all of the studied colorless antibiotic pollutants. For instance, the reaction rate kinetic constant of the g-C3N4@Cs0.33WO3 heterojunction is about 2.7 times faster than that of g-C3N4 and 4.6 times faster than that of Cs0.33WO3 for the photodegradation of ciprofloxacin under the same experimental conditions.
Table 5. Pseudo-First-Order Rate Constants (min–1) for Enrofloxacin, Ciprofloxacin, and TC Degradation under Solar Light Irradiation.
|
k (10–3 min–1) |
|||
|---|---|---|---|
| photocatalyst | enrofloxacin | ciprofloxacin | TC |
| g-C3N4 | 8.0 | 5.5 | 11.6 |
| Cs0.33WO3 | 7.0 | 3.2 | 5.3 |
| g-C3N4@Cs0.33WO3 | 15.2 | 14.9 | 14.3 |
The stability and reusability of the developed photocatalyst, which are crucial for practical applications,57 were studied, and the results in Figure 8a,b show that the synthesized g-C3N4@Cs0.33WO3 heterojunction exhibited significant stability for the photocatalytic degradation of the cationic dye Rh B and anionic dye MO for four consecutive experiments. There was no significant decrease in the photocatalytic performance observed in the four cycles for pollutants, indicating the stability and reusability of the developed photocatalyst. These findings show that the g-C3N4@Cs0.33WO3 heterojunction possesses excellent stability for the photocatalytic degradation of cationic and anionic dyes and can be a promising candidate for practical application in environmental remediation.
Figure 8.

Cyclic stability tests for (a) Rh B and (b) MO degradation using g-C3N4@Cs0.33WO3 under solar light irradiation.
3.5. Effect of Operational Parameters on Photocatalytic Properties
3.5.1. Effect of pH on the Photocatalytic Performance
The effects of pH on the photocatalytic performance of the developed g-C3N4@Cs0.33WO3 heterojunction was investigated, and the findings are presented in Figure 9a,b. The result shows that the pH has a significant effect on the photocatalytic performance of the developed catalyst and the highest photodegradation efficiency (97%) was achieved for TC at pH 3 (Figure 9a). According to previous reports, TC exists mainly in three different forms based on the pH of the solution. At the more acidic pH < 3.3, it exists as TCH3+. When the solution pH is between 3.3 and 7.7, it exists as neutral TCH2. For a solution of pH > 7.7 it exists as an anion, TCH– or TCH2–.70 Therefore, the adsorption of TC on the surface of the g-C3N4@Cs0.33WO3 heterojunction decreased with an increase in the pH of the solution. This was due to the fact that above pH 7 TC is negatively charged and the catalyst charge is also negative. There is a high repulsion interaction between the similar charges. These results decrease the TC degradation efficiency. Moreover, the effect of pH on the photodegradation of Rh B was also studied, and the result in Figure 9b depicts that the greatest amount of Rh B (99%) can be photodegraded at pH 3. This is due to the strong adsorption of the cationic form of Rh B onto the photocatalyst at pH 3 that facilitates the photodegradation efficiency.71 The ζ potential analysis result shown in Figure S3 also indicates that the developed g-C3N4@Cs0.33WO3 heterojunction possesses a negative surface charge and the electrostatic attraction between the negatively charged photocatalyst and positively charged pollutants at lower pH facilitates fast photodegradation at pH 3.
Figure 9.

Effect of pH on the photodegradation of (a) TC and (b) Rh B. (c) Effect of catalyst dosage and (d) dye concentration on the photodegradation of Rh B.
3.5.2. Effect of Concentration on Photocatalytic Properties
The effect of the pollutant concentration on the photocatalytic ability of the catalyst was also evaluated, and the finding in Figure 9c shows that the photodegradation efficiency decreases as the concentration of Rh B increases. The decrease in photodegradation efficiency of the photocatalyst with an increase in pollutant concentration can be due to presence of more pollutant molecules than accessible active sites on the photocatalyst. Besides, theexistence of more dye molecules at higher dye concentrations can also decrease the amount of light that reaches the photocatalyst surface and further decreases the photocatalytic performance.72,73 Since a 20 ppm dye concentration presented the highest photodegradation efficiency, it was selected for further study.
3.5.3. Effect of Catalyst Dosage on Photocatalytic Performance
The effect of catalyst dosage on the photodegradation of Rh B was studied by varying the amounts of catalyst from 10 to 20, 50, and 100 mg while keeping other parameters constant. Figure 9d shows that the photodegradation efficiency of Rh B sharply increased to 99% within 120 min as the catalyst dosage increased from 10 to 20 mg. However, further increments of photocatalyst dosage deceases the photodegradation efficiency. This negative effect at higher amounts of catalyst dosage is due to aggregation, photon scattering, suspension turbidity, and masking of the photosensitive surface, and thus, the hindrance of photon penetration in the solid phase decreases the photocatalytic activity.74,75
3.6. Identification of Intermediates and Possible Photocatalytic Mechanism
The intermediate products of the photodegradation of TC, ciprofloxacin, and enrofloxacin were investigated by using GC-MS. For the photodegradation of TC, three intermediates (Table S1) were identified based on the molecular ions in the MS spectrum.76,77 The chromatograms of the identified intermediates are shown in Figure S4. From the finding, it can be concluded that the TC photodegradation process takes place through dehydoxylation, N-demethylation, deamination, and ring opening in which the carbon–carbon bonds of TC are attacked by superoxide and hydroxyl radicals that leads to the generation of intermediates with carbonyl or hydroxyl groups.78 The intermediate products of the ciprofloxacin photodegradation were also studied, and the GC-MS spectrum in Figure S5 presents the formation of three different intermediates during the photocatalysis reaction (Table S2). Those intermediate formations can be ascribed to cleavage of the C–C and C–N bonds.79 During the photodegradation of ciprofloxacin, piperazine ring cleavage and decarboxylation of the quinolone ring takes place due to the attack of superoxide radicals and finally the intermediates can be converted to small molecules such as H2O, F–, and CO2.80 Moreover, the byproducts of the enrofloxacin were also investigated, and the results given in Figure S6 and Table S3 depict that six intermediates were generated during the photodegradation of enrofloxacin. The findings reveal that the photodegradation of enrofloxacin takes place via defluorination, cyclopropane and piperazine bond cleavage, and benzene and quinolone ring cleavage from the attack of photogenerated active species such as superoxide radicals, holes, and hydroxyl radicals. Then, the intermediates mineralized to smaller molecules such as NH4+, H2O, and CO2.81−83
To propose a photocatalytic mechanism for the photodegradation of the above pollutants, the roles of superoxide radicals, hydroxyl radicals, and holes were investigated using the respective scavengers BQ, IPA, and EDTA on the photodegradation of MO and TC. Figure 10a shows that the photodegradation of MO was largely suppressed in the presence of IPA followed by EDTA and was slightly affected by BQ, which indicates that hydroxyl radicals play a major role in the photodegradation of MO followed by holes and superoxide radicals, respectively. In the case of TC photodegradation (Figure 10b), the presence of BQ largely suppressed the photodegradation and was sequentially followed by EDTA and IPA, which showed that superoxide radicals play a dominant role in the photodegradation, followed by holes and hydroxyl radicals, respectively.
Figure 10.

Active species determination for (a) MO and (b) TC.
Based on the above results, a possible photocatalytic mechanism for the developed g-C3N4@Cs0.33WO3 heterojunction can be proposed, as depicted in Figure 11. Accordingly, when solar light is irradiated on the photocatalyst, electrons are excited from the VBs of both materials to their respective CBs due to their suitable band gaps and simultaneously leave holes in their respective VBs.6 These photogenerated electrons and holes move to the surface and are responsible for photocatalysis. These photogenerated charge carriers produce reactive oxygen species (ROS), such as superoxide radicals and hydroxyl radicals, that have sufficient energies to decompose organic contaminants.6 The irradiation of solar light will cause the excitation of electrons from the VBs of g-C3N4 and Cs0.33WO3, which leaves holes in the VBs (eq 4). Thereafter, the electrons in the CB of g-C3N4 transfer to the CB of Cs0.33WO3. Subsequently, reaction with dissolved oxygen at the surface and formation of superoxide radicals (eq 5) cause pollutant degradation, whereas the holes in the VB of Cs0.33WO3 transferring to the VB of g-C3N4 directly react with the pollutant and possess sufficient energy to degrade organic pollutants.26,57 Thus, the generated charge carriers are responsible for the overall photocatalytic degradation of the pollutants and the heterojunction formation could enhance the separation of photogenerated charge carriers compared to pure materials, thereby resulting in an improved photocatalytic activity.7
| 4 |
| 5 |
| 6 |
| 7 |
Figure 11.

Proposed photocatalytic mechanism for the photocatalytic activity of g-C3N4@Cs0.33WO3 in the photodegradation of TC.
4. Conclusions
In this study, we successfully synthesized a solar light driven g-C3N4@Cs0.33WO3 heterojunction by a solvothermal method. The developed g-C3N4@Cs0.33WO3 heterojunction photocatalyst exhibited an excellent photocatalytic performance toward colored cationic and anionic organic dyes as well as colorless enrofloxacin, ciprofloxacin, and TC antibiotics. The photocatalytic activities of g-C3N4@Cs0.33WO3 heterojunctions toward the photodegradation of MO, Rh B, MB, NR, enrofloxacin, ciprofloxacin, and TC were approximately 4.5, 12, 5, 3, 2.1, 4.6, and 2.7 times faster than those of pure Cs0.33WO3, respectively. This indicates that the fabricated photocatalyst can be utilized as a universal photocatalyst for the treatment of different wastewater pollutants. The developed photocatalyst also exhibited photocatalytic performance superior to those of reported solar light active photocatalysts for the photodegradation of Rh B and TC. Therefore, it is an excellent photocatalyst for the photodegradation of organic dyes and colorless antibiotics under solar light. The trapping experiments revealed that superoxide radicals, hydroxyl radicals, and holes play crucial roles in determining the photocatalytic activity of the fabricated photocatalyst. Among all pollutants studied in this work, the cationic dye MB photodegraded the fastest compared to others: 98.2% of it was photodegraded within just 1 h. The result shows that the g-C3N4@Cs0.33WO3 heterojunction exhibits highest adsorption toward MB in comparison to other pollutants and this high adsorption property of the MB on the developed photocatalyst facilitated the fast photodegradation of the pollutant. The effective photocatalytic performance of the developed g-C3N4@Cs0.33WO3 could be ascribed to the extended full-spectrum light absorption, high surface area, effective charge separation of photogenerated charge carriers, and narrow band gap. Therefore, this study paves the way for environmental scientists to design an effective, low-cost, and efficient solar light active photocatalyst for environmental remediation.
Acknowledgments
The authors thank the Ministry of Science and Technology (MOST), Taiwan, Republic of China, for financial support under contract number MOST110-2811-E-011-505-MY3.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03675.
Identified intermediates, kinetic pseudo-first-order plots, ζ potential, and GC-MS spectra of enrofloxacin, TC, and ciprofloxacin antibiotics (PDF)
Author Contributions
A.A.T.: investigation, data curation, and writing. C.-M.W.: conceptualization, supervision, review, and editing. K.G.M.: review, editing, methodology, and investigation.
The authors declare no competing financial interest.
Supplementary Material
References
- Nethravathi C.; Rajamathi J. T.; Rajamathi M. Microwave-assisted synthesis of porous aggregates of CuS nanoparticles for sunlight photocatalysis. ACS omega 2019, 4 (3), 4825–4831. 10.1021/acsomega.8b03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi Y.; Nagakawa H.; Nagata M. Highly Efficient Photocatalytic Degradation of Hydrogen Sulfide in the Gas Phase Using Anatase/TiO2 (B) Nanotubes. ACS omega 2022, 7 (14), 11946–11955. 10.1021/acsomega.1c07294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Liu B.; Xu J.; Wang Q.; Wang X.; Lv G.; Zhou J. The Synthesis of h-BN-Modified Z-Scheme WO3/g-C3N4 Heterojunctions for Enhancing Visible Light Photocatalytic Degradation of Tetracycline Pollutants. ACS omega 2022, 7 (7), 6035–6045. 10.1021/acsomega.1c06377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobadifard M.; Mohebbi S.; Radovanovic P. V. Selective oxidation of alcohols by using CoFe2O4/Ag2MoO4 as a visible-light-driven heterogeneous photocatalyst. New J. Chem. 2020, 44 (7), 2858–2867. 10.1039/C9NJ05633E. [DOI] [Google Scholar]
- Kohantorabi M.; Hosseinifard M.; Kazemzadeh A. Catalytic activity of a magnetic Fe2O3@CoFe2O4 nanocomposite in peroxymonosulfate activation for norfloxacin removal. New J. Chem. 2020, 44 (10), 4185–4198. 10.1039/C9NJ04379A. [DOI] [Google Scholar]
- Motora K. G.; Wu C.-M.; Chala T. F.; Chou M.-H.; Kuo C.-F. J.; Koinkar P. Highly efficient photocatalytic activity of Ag3VO4/WO2.72 nanocomposites for the degradation of organic dyes from the ultraviolet to near-infrared regions. Appl. Surf. Sci. 2020, 512, 145618. 10.1016/j.apsusc.2020.145618. [DOI] [Google Scholar]
- Motora K. G.; Wu C.-M.; Naseem S. Magnetic recyclable self-floating solar light-driven WO2.72/Fe3O4 nanocomposites immobilized by Janus membrane for photocatalysis of inorganic and organic pollutants. J. Ind. Eng. Chem. 2021, 102, 25–34. 10.1016/j.jiec.2021.06.025. [DOI] [Google Scholar]
- Wang H.; Yuan X.; Wu Y.; Zeng G.; Dong H.; Chen X.; Leng L.; Wu Z.; Peng L. In situ synthesis of In2S3@ MIL-125 (Ti) core–shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl. Catal. 2016, 186, 19–29. 10.1016/j.apcatb.2015.12.041. [DOI] [Google Scholar]
- Motora K. G.; Wu C.-M.; Xu T.-Z.; Chala T. F.; Lai C.-C. Photocatalytic, antibacterial, and deodorization activity of recycled triacetate cellulose nanocomposites. Mater. Chem. Phys. 2020, 240, 122260. 10.1016/j.matchemphys.2019.122260. [DOI] [Google Scholar]
- Purnachander Rao M.; Wu J. J.; Asiri A. M.; Anandan S. Rice grain like Bi2S3 nanorods and its photocatalytic performance. Mater. Sci. Eng., B 2021, 268, 115144. 10.1016/j.mseb.2021.115144. [DOI] [Google Scholar]
- Habibi-Yangjeh A.; Asadzadeh-Khaneghah S.; Feizpoor S.; Rouhi A. Review on heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: Can we win against pathogenic viruses?. J. Colloid Interface Sci. 2020, 580, 503–514. 10.1016/j.jcis.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Hu C.; Xi Y.; Wan B.; Zhang C.; Zhang Y. Synthesis and visible light photocatalytic activity of β-AgVO3 nanowires. Solid State Sci. 2012, 14 (4), 535–539. 10.1016/j.solidstatesciences.2012.01.013. [DOI] [Google Scholar]
- Huang C.; Wang J.; Li M.; Lei X.; Wu Q. Construction of a novel Z-scheme V2O5/NH2-MIL-101(Fe)composite photocatalyst with enhanced photocatalytic degradation of tetracycline. Solid State Sci. 2021, 117, 106611. 10.1016/j.solidstatesciences.2021.106611. [DOI] [Google Scholar]
- Ming J.; Liu N.; Ma Q.; Sharma A.; Sun X.; Kawazoe N.; Chen G.; Yang Y. Bactericidal process and practicability for environmental water sterilization by solar-light-driven Bi2WO6-based photocatalyst. J. Water Process. Eng. 2022, 47, 102713. 10.1016/j.jwpe.2022.102713. [DOI] [Google Scholar]
- Shibu M. C.; Benoy M. D.; Shanavas S.; Haija M. A.; Duraimurugan J.; Kumar G. S.; Ahamad T.; Maadeswaran P.; Van Le Q. White LED active α-Fe2O3/rGO photocatalytic nanocomposite for an effective degradation of tetracycline and ibuprofen molecules. Environ. Res. 2022, 212, 113301. 10.1016/j.envres.2022.113301. [DOI] [PubMed] [Google Scholar]
- Chala T. F.; Wu C.-M.; Motora K. G. RbxWO3/Ag3VO4 nanocomposites as efficient full-spectrum (UV, visible, and near-infrared) photocatalysis. J. Taiwan Inst Chem. Eng. 2019, 102, 465–474. 10.1016/j.jtice.2019.06.016. [DOI] [Google Scholar]
- Ademola Bode-Aluko C.; Pereao O.; Kyaw H. H.; Al-Naamani L.; Al-Abri M. Z.; Tay Zar Myint M.; Rossouw A.; Fatoba O.; Petrik L.; Dobretsov S. Photocatalytic and antifouling properties of electrospun TiO2 polyacrylonitrile composite nanofibers under visible light. Mater. Sci. Eng., B 2021, 264, 114913. 10.1016/j.mseb.2020.114913. [DOI] [Google Scholar]
- Chen Z.; Cheng C.; Xing F.; Huang C. Strong interfacial coupling for NiS thin layer covered CdS nanorods with highly efficient photocatalytic hydrogen production. New J. Chem. 2020, 44 (44), 19083–19090. 10.1039/D0NJ04335D. [DOI] [Google Scholar]
- Piranshahi Z. A.; Behbahani M.; Zeraatpisheh F. Synthesis, characterization and photocatalytic application of TiO2/magnetic graphene for efficient photodegradation of crystal violet. Appl. Organomet. Chem. 2018, 32 (1), e3985 10.1002/aoc.3985. [DOI] [Google Scholar]
- Salimi M.; Behbahani M.; Sobhi H. R.; Gholami M.; Jafari A. J.; Kalantary R. R.; Farzadkia M.; Esrafili A. A new nano-photocatalyst based on Pt and Bi co-doped TiO2 for efficient visible-light photo degradation of amoxicillin. New J. Chem. 2019, 43 (3), 1562–1568. 10.1039/C8NJ05020A. [DOI] [Google Scholar]
- Salimi M.; Esrafili A.; Sobhi H. R.; Behbahani M.; Gholami M.; Farzadkia M.; Jafari A. J.; Kalantary R. R. Photocatalytic Degradation of Metronidazole Using D-g-C3N4-Bi5O7I Composites Under Visible Light Irradiation: Degradation Product, and Mechanisms. Chemistry Select 2019, 4 (35), 10288–10295. 10.1002/slct.201902369. [DOI] [Google Scholar]
- Pourzad A.; Sobhi H. R.; Behbahani M.; Esrafili A.; Kalantary R. R.; Kermani M. Efficient visible light-induced photocatalytic removal of paraquat using N-doped TiO2@SiO2@Fe3O4 nanocomposite. J. Mol. Liq. 2020, 299, 112167. 10.1016/j.molliq.2019.112167. [DOI] [Google Scholar]
- Abilarasu A.; Kumar P. S.; Vo D.-V. N.; Krithika D.; Ngueagni P. T.; Joshiba G. J.; Carolin C. F.; Prasannamedha G. J. Enhanced photocatalytic degradation of diclofenac by Sn0.15Mn0.85Fe2O4 catalyst under solar light. J. Environ. Chem. Eng. 2021, 9 (1), 104875. 10.1016/j.jece.2020.104875. [DOI] [Google Scholar]
- Naseem S.; Wu C.-M.; Motora K. G. Novel multifunctional RbxWO3@Fe3O4 immobilized Janus membranes for desalination and synergic-photocatalytic water purification. Desalination 2021, 517, 115256. 10.1016/j.desal.2021.115256. [DOI] [Google Scholar]
- Tessema A. A.; Wu C.-M.; Motora K. G.; Naseem S. Highly-efficient and salt-resistant CsxWO3@g-C3N4/PVDF fiber membranes for interfacial water evaporation, desalination, and sewage treatment. Compos. Sci. Technol. 2021, 211, 108865. 10.1016/j.compscitech.2021.108865. [DOI] [Google Scholar]
- Gu J.-w.; Guo R.-t.; Miao Y.-f.; Liu Y.-z.; Wu G.-l.; Duan C.-p.; Pan W.-g. Construction of full spectrum-driven CsxWO3/g-C3N4 heterojunction catalyst for efficient photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 540, 148316. 10.1016/j.apsusc.2020.148316. [DOI] [Google Scholar]
- Shrisha; Wu C.-M.; Motora K. G.; Kuo D.-H.; Lai C.-C.; Huang B.-R.; Saravanan A. Cesium tungsten bronze nanostructures and their highly enhanced hydrogen gas sensing properties at room temperature. Int. J. Hydrog. Energy 2021, 46 (50), 25752–25762. 10.1016/j.ijhydene.2021.05.064. [DOI] [Google Scholar]
- Motora K. G.; Wu C. M.; Chang C. C.; Liao J. H. NIR Light Stimulated Self-Healing Reduced Tungsten Oxide/Polyurethane Nanocomposite Based on the Diels– Alder Reaction. Macromol. Mater. Eng. 2021, 306, 2100438. 10.1002/mame.202100438. [DOI] [Google Scholar]
- Gong J.; Xie Z.; Wang B.; Li Z.; Zhu Y.; Xue J.; Le Z. Fabrication of g-C3N4-based conjugated copolymers for efficient photocatalytic reduction of U(VI). J. Environ. Chem. Eng. 2021, 9 (1), 104638. 10.1016/j.jece.2020.104638. [DOI] [Google Scholar]
- Akhundi A.; Habibi-Yangjeh A.; Abitorabi M.; Rahim Pouran S. Review on photocatalytic conversion of carbon dioxide to value-added compounds and renewable fuels by graphitic carbon nitride-based photocatalysts. Catal. Rev. Sci. Eng. 2019, 61 (4), 595–628. 10.1080/01614940.2019.1654224. [DOI] [Google Scholar]
- Akhundi A.; Badiei A.; Ziarani G. M.; Habibi-Yangjeh A.; Muñoz-Batista M. J.; Luque R. Graphitic carbon nitride-based photocatalysts: Toward efficient organic transformation for value-added chemicals production. Mol. Catal. 2020, 488, 110902. 10.1016/j.mcat.2020.110902. [DOI] [Google Scholar]
- Asadzadeh-Khaneghah S.; Habibi-Yangjeh A. g-C3N4/carbon dot-based nanocomposites serve as efficacious photocatalysts for environmental purification and energy generation: A review. J. Clean. Prod. 2020, 276, 124319. 10.1016/j.jclepro.2020.124319. [DOI] [Google Scholar]
- Zhang X.; Wang X.; Meng J.; Liu Y.; Ren M.; Guo Y.; Yang Y. Robust Z-scheme g-C3N4/WO3 heterojunction photocatalysts with morphology control of WO3 for efficient degradation of phenolic pollutants. Sep. Purif. Technol. 2021, 255, 117693. 10.1016/j.seppur.2020.117693. [DOI] [Google Scholar]
- Zhang Y.; Zhou J.; Feng Q.; Chen X.; Hu Z. Visible light photocatalytic degradation of MB using UiO-66/g-C3N4 heterojunction nanocatalyst. Chemosphere 2018, 212, 523–532. 10.1016/j.chemosphere.2018.08.117. [DOI] [PubMed] [Google Scholar]
- Han C.; Ge L.; Chen C.; Li Y.; Xiao X.; Zhang Y.; Guo L. Novel visible light induced Co3O4-g-C3N4 heterojunction photocatalysts for efficient degradation of methyl orange. Appl. Catal. 2014, 147, 546–553. 10.1016/j.apcatb.2013.09.038. [DOI] [Google Scholar]
- Li N.; Fan H.; Dai Y.; Kong J.; Ge L. Insight into the solar utilization of a novel Z-scheme Cs0.33WO3/CdS heterostructure for UV–Vis-NIR driven photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 508, 145200. 10.1016/j.apsusc.2019.145200. [DOI] [Google Scholar]
- Huang X.; Liu J.-X.; Shi F.; Yu L.; Liu S.-H. Ambient pressure drying synthesis of Cs0.33WO3/SiO2 composite aerogels for efficient removal of Rhodamine B from water. Mater. Des. 2016, 110, 624–632. 10.1016/j.matdes.2016.08.031. [DOI] [Google Scholar]
- Wu X.; Yin S.; Xue D.; Komarneni S.; Sato T. A CsxWO3/ZnO nanocomposite as a smart coating for photocatalytic environmental cleanup and heat insulation. Nanoscale 2015, 7 (40), 17048–17054. 10.1039/C5NR04452A. [DOI] [PubMed] [Google Scholar]
- Li N.; Gao X.; Fan H.; Gao Y.; Ge L. Insight into the relationship of the high photocatalytic performance and double photochromic activity of Z-scheme CsxWO3/AgBr heterostructures under UV–Vis-NIR light utilization. Appl. Surf. Sci. 2020, 529, 147038. 10.1016/j.apsusc.2020.147038. [DOI] [Google Scholar]
- Zhu B.; Xia P.; Li Y.; Ho W.; Yu J. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N4/Ag2WO4 photocatalyst. Appl. Surf. Sci. 2017, 391, 175–183. 10.1016/j.apsusc.2016.07.104. [DOI] [Google Scholar]
- Chen M.; Guo C.; Hou S.; Lv J.; Zhang Y.; Zhang H.; Xu J. A novel Z-scheme AgBr/P-g-C3N4 heterojunction photocatalyst: Excellent photocatalytic performance and photocatalytic mechanism for ephedrine degradation. Appl. Catal. 2020, 266, 118614. 10.1016/j.apcatb.2020.118614. [DOI] [Google Scholar]
- Huang J.; Li D.; Li R.; Chen P.; Zhang Q.; Liu H.; Lv W.; Liu G.; Feng Y. One-step synthesis of phosphorus/oxygen co-doped g-C3N4/anatase TiO2 Z-scheme photocatalyst for significantly enhanced visible-light photocatalysis degradation of enrofloxacin. J. Hazard. Mater. 2020, 386, 121634. 10.1016/j.jhazmat.2019.121634. [DOI] [PubMed] [Google Scholar]
- Ardani M. R.; Pang A. L.; Pal U.; Zheng R.; Arsad A.; Hamzah A. A.; Ahmadipour M. Ultrasonic-assisted polyaniline-multiwall carbon nanotube photocatalyst for efficient photodegradation of organic pollutants. J. Water Process. Eng. 2022, 46, 102557. 10.1016/j.jwpe.2021.102557. [DOI] [Google Scholar]
- Chiam S.-L.; Pung S.-Y.; Yeoh F. Y.; Ahmadipour M. Highly efficient oxidative degradation of organic dyes by manganese dioxide nanoflowers. Mater. Chem. Phys. 2022, 280, 125848. 10.1016/j.matchemphys.2022.125848. [DOI] [Google Scholar]
- Ahmadipour M.; Hamzah A. A.; Pang A. L.; Thi Le A.; Chiam S.-L.; Ahmad Z. A.; Rajitha B.; Pung S. Y. Photodegradation of rhodamine B-dye pollutant using CaCu3Ti4O12-multiwall carbon nanotube nanocomposites. J. Environ. Chem. Eng. 2021, 9 (3), 105185. 10.1016/j.jece.2021.105185. [DOI] [Google Scholar]
- Bekena F. T.; Kuo D.-H.; Kebede W. L. Universal and highly efficient degradation performance of novel Bi2(O,S)3/Mo(O,S)2 nanocomposite photocatalyst under visible light. Sep. Purif. Technol. 2020, 247, 117042. 10.1016/j.seppur.2020.117042. [DOI] [Google Scholar]
- Khasawneh O. F. S.; Palaniandy P.; Ahmadipour M.; Mohammadi H.; Bin Hamdan M. R. Removal of acetaminophen using Fe2O3-TiO2 nanocomposites by photocatalysis under simulated solar irradiation: Optimization study. J. Environ. Chem. Eng. 2021, 9 (1), 104921. 10.1016/j.jece.2020.104921. [DOI] [Google Scholar]
- Chiam S.-L.; Soo Q.-Y.; Pung S.-Y.; Ahmadipour M. Polycrystalline TiO2 particles synthesized via one-step rapid heating method as electrons transfer intermediate for Rhodamine B removal. Mater. Chem. Phys. 2021, 257, 123784. 10.1016/j.matchemphys.2020.123784. [DOI] [Google Scholar]
- Ahmadipour M.; Arjmand M.; Thirmizir M. Z. A.; Le A. T.; Chiam S. L.; Pung S.-Y. Synthesis of core/shell-structured CaCu3Ti4O12/SiO2 composites for effective degradation of rhodamine B under ultraviolet light. J. Mater. Sci. Mater. Electron. 2020, 31 (22), 19587–19598. 10.1007/s10854-020-04486-1. [DOI] [Google Scholar]
- Ahmadipour M.; Arjmand M.; Ahmad Z. A.; Pung S.-Y. Photocatalytic Degradation of Organic Dye by Sol–Gel-Synthesized CaCu3Ti4O12 Powder. J. Mater. Eng. Perform. 2020, 29 (3), 2006–2014. 10.1007/s11665-020-04712-1. [DOI] [Google Scholar]
- Ahmadipour M.; Arjmand M.; Qurratu Aini Abd Aziz S. N.; Chiam S. L.; Ahmad Z. A.; Pung S.-Y. Influence of annealing temperature on morphological and photocatalytic activity of sputter-coated CaCu3Ti4O12 thin film under ultraviolet light irradiation. Ceram. Int. 2019, 45 (16), 20697–20703. 10.1016/j.ceramint.2019.07.053. [DOI] [Google Scholar]
- Tang R.; Gong D.; Deng Y.; Xiong S.; Zheng J.; Li L.; Zhou Z.; Su L.; Zhao J. π-π stacking derived from graphene-like biochar/g-C3N4 with tunable band structure for photocatalytic antibiotics degradation via peroxy monosulfate activation. Hazard. Mater. 2022, 423, 126944. 10.1016/j.jhazmat.2021.126944. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zhu G.; Hojamberdiev M.; Gao J.; Zhu R.; Wang C.; Wei X.; Liu P. Three-dimensional Ag2O/Bi5O7I p–n heterojunction photocatalyst harnessing UV–vis–NIR broad spectrum for photodegradation of organic pollutants. J. Hazard. Mater. 2018, 344, 42–54. 10.1016/j.jhazmat.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Bekena F. T.; Kuo D.-H.; Kebede W. L. Universal and highly efficient degradation performance of novel Bi2(O,S)3/Mo(O,S)2 nanocomposite photocatalyst under visible light. Sep. Purif. Technol. 2020, 247, 117042. 10.1016/j.seppur.2020.117042. [DOI] [Google Scholar]
- Chen C.; Li M.; Jia Y.; Chong R.; Xu L.; Liu X. Surface defect-engineered silver silicate/ceria pn heterojunctions with a flower-like structure for boosting visible light photocatalysis with mechanistic insight. J. Colloid Interface Sci., 2020, 564, 442–453. 10.1016/j.jcis.2019.12.128. [DOI] [PubMed] [Google Scholar]
- Ren X.; Zhang X.; Guo R.; Li X.; Peng Y.; Zhao X.; Pu X. P. Hollow mesoporous g-C3N4/Ag2CrO4 photocatalysis with direct Z-scheme: Excellent degradation performance for antibiotics and dyes. Sep. Purif. Technol. 2021, 270, 118797. 10.1016/j.seppur.2021.118797. [DOI] [Google Scholar]
- Motora K. G.; Wu C.-M. Magnetically separable highly efficient full-spectrum light-driven WO2.72/Fe3O4 nanocomposites for photocatalytic reduction of carcinogenic chromium (VI) and organic dye degradation. J. Taiwan Inst Chem. Eng. 2020, 117, 123–132. 10.1016/j.jtice.2020.12.006. [DOI] [Google Scholar]
- Palanisamy G.; Bhuvaneswari K.; Chinnadurai A.; Bharathi G.; Pazhanivel T. Magnetically recoverable multifunctional ZnS/Ag/CoFe2O4 nanocomposite for sunlight driven photocatalytic dye degradation and bactericidal application. J. Phys. Chem. Solids 2020, 138, 109231. 10.1016/j.jpcs.2019.109231. [DOI] [Google Scholar]
- Singla S.; Sharma S.; Basu S. P. MoS2/WO3 heterojunction with the intensified photocatalytic performance for decomposition of organic pollutants under the broad array of solar light. J. Clean. Prod. 2021, 324, 129290. 10.1016/j.jclepro.2021.129290. [DOI] [Google Scholar]
- Samanta D.; Chanu T. I.; Basnet P.; Chatterjee S. J. Organic dye degradation under solar irradiation by hydrothermally synthesized ZnS nanospheres. J. Mater. Eng. Perform., 2018, 27 (6), 2673–2678. 10.1007/s11665-018-3214-0. [DOI] [Google Scholar]
- Xiong T.; Ye Y.; Luo B.; Shen L.; Wang D.; Fan M.; Gong Z. Facile fabrication of 3D TiO2-graphene aerogel composite with enhanced adsorption and solar light-driven photocatalytic activity. Ceram. Int. 2021, 47 (10), 14290–14300. 10.1016/j.ceramint.2021.02.011. [DOI] [Google Scholar]
- Ji Z.; Callahan D. M.; Ismail M. N.; Warzywoda J.; Sacco A. Development and characterization of a titanosilicate ETS-10-coated optical fiber reactor towards the photodegradation of methylene blue. J. Photochem. Photobiol. 2011, 217 (1), 22–28. 10.1016/j.jphotochem.2010.09.011. [DOI] [Google Scholar]
- Ramanathan S.; Moorthy S.; Ramasundaram S.; Rajan H. K.; Vishwanath S.; Selvinsimpson S.; Durairaj A.; Kim B.; Vasanthkumar S. Grape Seed Extract Assisted Synthesis of Dual-Functional Anatase TiO2 Decorated Reduced Graphene Oxide Composite for Supercapacitor Electrode Material and Visible Light Photocatalytic Degradation of Bromophenol Blue Dye. ACS Omega 2021, 6 (23), 14734–14747. 10.1021/acsomega.0c02325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalponte Dallabona I.; Mathias Á. L.; Jorge R. M. M. A new green floating photocatalyst with Brazilian bentonite into TiO2/alginate beads for dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127159. 10.1016/j.colsurfa.2021.127159. [DOI] [Google Scholar]
- Yu X.; He J.; Zhang Y.; Hu J.; Chen F.; Wang Y.; He G.; Liu J.; He Q. Effective photodegradation of tetracycline by narrow-energy band gap photocatalysts La2-xSrxNiMnO6 (x = 0, 0.05, 0.10, and 0.125). J. Alloys Compd. 2019, 806, 451–463. 10.1016/j.jallcom.2019.07.233. [DOI] [Google Scholar]
- Lu Q.; Dong C.; Wei F.; Li J.; Wang Z.; Mu W.; Han X. Rational fabrication of Bi2WO6 decorated TiO2 nanotube arrays for photocatalytic degradation of organic pollutants. Mater. Res. Bull. 2022, 145, 111563. 10.1016/j.materresbull.2021.111563. [DOI] [Google Scholar]
- Cao J.; Yang Z.-h.; Xiong W.-p.; Zhou Y.-y.; Peng Y.-r.; Li X.; Zhou C.-y.; Xu R.; Zhang Y.-r. One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis. Chem. Eng. Sci. 2018, 353, 126–137. 10.1016/j.cej.2018.07.060. [DOI] [Google Scholar]
- Han W.; Wu T.; Wu Q. I. Fabrication of WO3/Bi2MoO6 heterostructures with efficient and highly selective photocatalytic degradation of tetracycline hydrochloride. J. Colloid Interface Sci. 2021, 602, 544–552. 10.1016/j.jcis.2021.05.128. [DOI] [PubMed] [Google Scholar]
- Nasseh N.; Barikbin B.; Taghavi L. Innovation, Photocatalytic degradation of tetracycline hydrochloride by FeNi3/SiO2/CuS magnetic nanocomposite under simulated solar irradiation: Efficiency, stability, kinetic and pathway study. Environ. Technol. Innov. 2020, 20, 101035. 10.1016/j.eti.2020.101035. [DOI] [Google Scholar]
- Tang R.; Gong D.; Deng Y.; Xiong S.; Zheng J.; Li L.; Zhou Z.; Su L.; Zhao J. π-π stacking derived from graphene-like biochar/g-C3N4 with tunable band structure for photocatalytic antibiotics degradation via peroxymonosulfate activation. J. Hazard. Mater. 2022, 423, 126944. 10.1016/j.jhazmat.2021.126944. [DOI] [PubMed] [Google Scholar]
- Sakthivel S.; Neppolian B.; Shankar M. V.; Arabindoo B.; Palanichamy M.; Murugesan V. Solar photocatalytic degradation of azo dye: comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77 (1), 65–82. 10.1016/S0927-0248(02)00255-6. [DOI] [Google Scholar]
- Taghavi Fardood S.; Moradnia F.; Forootan R.; Abbassi R.; Jalalifar S.; Ramazani A.; Sillanpää M. Facile green synthesis, characterization and visible light photocatalytic activity of MgFe2O4@CoCr2O4 magnetic nanocomposite. J. Photochem. Photobiol. 2022, 423, 113621. 10.1016/j.jphotochem.2021.113621. [DOI] [Google Scholar]
- Moradnia F.; Ramazani A.; Fardood S. T.; Gouranlou F. A novel green synthesis and characterization of tetragonal-spinel MgMn2O4 nanoparticles by tragacanth gel and studies of its photocatalytic activity for degradation of reactive blue 21 dye under visible light. Mater. Res. Express 2019, 6 (7), 075057. 10.1088/2053-1591/ab17bc. [DOI] [Google Scholar]
- Arabpour N.; Nezamzadeh-Ejhieh A. Modification of clinoptilolite nano-particles with iron oxide: Increased composite catalytic activity for photodegradation of cotrimaxazole in aqueous suspension. Mater. Sci. Semicond Process 2015, 31, 684–692. 10.1016/j.mssp.2014.12.067. [DOI] [Google Scholar]
- Ahmad M.; Qureshi M. T.; Rehman W.; Alotaibi N. H.; Gul A.; Abdel Hameed R. S.; Elaimi M. A.; Abd el-kader M. F. H.; Nawaz M.; Ullah R. Enhanced photocatalytic degradation of RhB dye from aqueous solution by biogenic catalyst Ag@ZnO. J. Alloys Compd. 2022, 895, 162636. 10.1016/j.jallcom.2021.162636. [DOI] [Google Scholar]
- Wang J.; Zhi D.; Zhou H.; He X.; Zhang D. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode. Water Res. 2018, 137, 324–334. 10.1016/j.watres.2018.03.030. [DOI] [PubMed] [Google Scholar]
- Jin J.; Liu M.; Feng L.; Wang H.; Wang Y.; Nguyen T. A.; Wang Y.; Lu J.; Li Y.; Bao M. 3D Bombax-structured carbon nanotube sponge coupling with Ag3PO4 for tetracycline degradation under ultrasound and visible light irradiation. Sci. Total Environ. 2019, 695, 133694. 10.1016/j.scitotenv.2019.133694. [DOI] [PubMed] [Google Scholar]
- Jin J.; Liu M.; Feng L.; Wang H.; Wang Y.; Nguyen T. A. H.; Wang Y.; Lu J.; Li Y.; Bao M. 3D Bombax-structured carbon nanotube sponge coupling with Ag3PO4 for tetracycline degradation under ultrasound and visible light irradiation. Sci. Total Environ. 2019, 695, 133694. 10.1016/j.scitotenv.2019.133694. [DOI] [PubMed] [Google Scholar]
- Manea Y. K.; Khan A. M.; Wani A. A.; Qashqoosh M. T. A.; Shahadat M.; Salem M. A. S. Hydrothermally synthesized mesoporous CS-g-PA@TSM functional nanocomposite for efficient photocatalytic degradation of Ciprofloxacin and treatment of metal ions. J. Mol. Liq. 2021, 335, 116144. 10.1016/j.molliq.2021.116144. [DOI] [Google Scholar]
- Chen F.; Yang Q.; Wang Y.; Yao F.; Ma Y.; Huang X.; Li X.; Wang D.; Zeng G.; Yu H. Efficient construction of bismuth vanadate-based Z-scheme photocatalyst for simultaneous Cr (VI) reduction and ciprofloxacin oxidation under visible light: Kinetics, degradation pathways and mechanism. Chem. Eng. J. 2018, 348, 157–170. 10.1016/j.cej.2018.04.170. [DOI] [Google Scholar]
- Wang Y.; Zhu C.; Zuo G.; Guo Y.; Xiao W.; Dai Y.; Kong J.; Xu X.; Zhou Y.; Xie A.; Sun C.; Xian Q. 0D/2D Co3O4/TiO2 Z-Scheme heterojunction for boosted photocatalytic degradation and mechanism investigation. Appl. Catal. 2020, 278, 119298. 10.1016/j.apcatb.2020.119298. [DOI] [Google Scholar]
- Lu Z.; Chen F.; He M.; Song M.; Ma Z.; Shi W.; Yan Y.; Lan J.; Li F.; Xiao P. Microwave synthesis of a novel magnetic imprinted TiO2 photocatalyst with excellent transparency for selective photodegradation of enrofloxacin hydrochloride residues solution. Chem. Eng. J. 2014, 249, 15–26. 10.1016/j.cej.2014.03.077. [DOI] [Google Scholar]
- Qiu W.; Zheng M.; Sun J.; Tian Y.; Fang M.; Zheng Y.; Zhang T.; Zheng C. Photolysis of enrofloxacin, pefloxacin and sulfaquinoxaline in aqueous solution by UV/H2O2, UV/Fe (II), and UV/H2O2/Fe (II) and the toxicity of the final reaction solutions on zebrafish embryos. Sci. Total Environ. 2019, 651, 1457–1468. 10.1016/j.scitotenv.2018.09.315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


