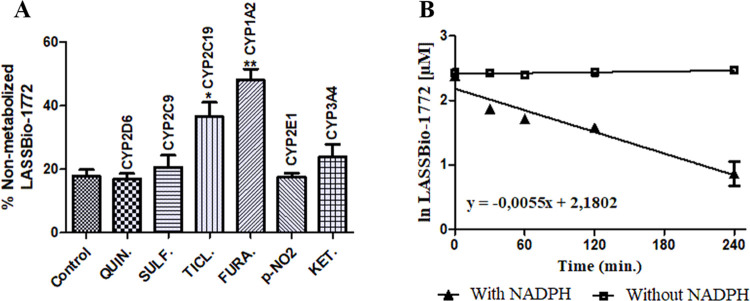
Figure 3.
Metabolism inhibition and experimental half-life of compound 2. (A) Enzymatic inhibition study using specific inhibitors of CYP isoenzymes to establish the enzymes responsible for in vitro hepatic microsomal metabolism of compound 2: quinidine (6 μM, CYP2D6), sulfaphenazole (6 μM, CYP2C9), ticlopidine (20 μM, CYP2C19), furafylline (40 μM, CYP1A2), p-nitrophenol (100 μM, CYP2E1), and ketoconazole (1 μM, CYP3A4). The biotransformation rate decreased about 50 and 35% with furafylline and ticlopidine, respectively, showing that CYP1A2 and CYP2C19 were responsible for metabolizing LASSBio-1772 (2). (B) Determination of half-life (t1/2= 126 min) based on the straight-line equation achieved by the natural logarithm of LASSBio-1772 concentration vs incubation time in the presence of NADPH. Data represent the mean of triplicate, performed with three different microsome samples (n = 9) after 240 min of incubation at 37 °C. **p-value < 0.0003; *p-value < 0.005. One-way ANOVA followed by Dunnett’s post-test, using GraphPrism software (version 5.0).