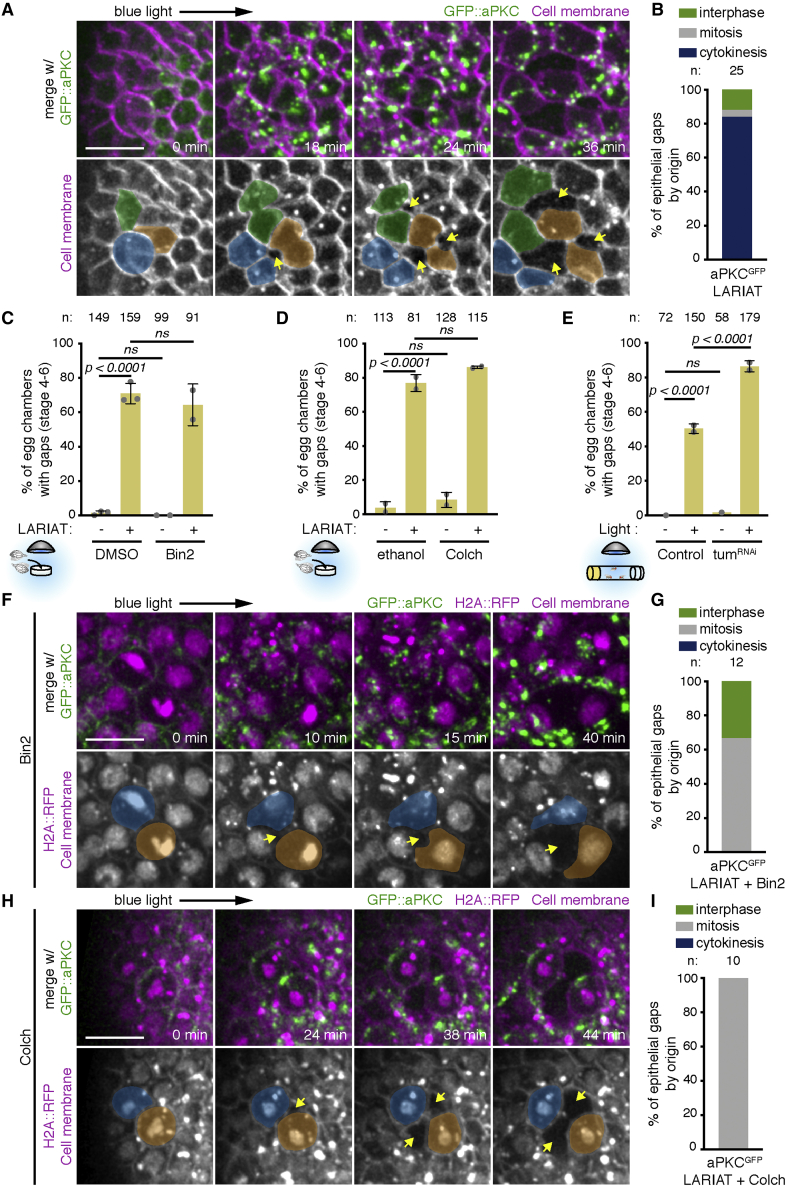
Figure 5.
Cell division challenges tissue cohesion upon aPKC inactivation
(A) Time-lapse images of an egg chamber (surface view) expressing LARIAT and GFP::aPKC and stained with membrane marker. Imaging with 488 nm laser clustered aPKC from min 0 onward. Epithelial gaps (arrows) form adjacent to dividing cells (colored).
(B) Quantification of epithelial-gap origin according to cell division stage of neighboring cells; n, number of gaps scored.
(C and D) Gap frequency in the presence or absence of LARIAT in egg chambers treated with Binucleine-2 (Bin-2) to inhibit AurB (C), or colchicine (Colch) to depolymerize microtubules (D), before light exposure for 2 h ex vivo.
(E) Frequency of epithelial gaps scored in control and TumRNAi egg chambers from flies expressing GFP::aPKC LARIAT and exposed (+) or not (−) to blue light for 2 h.
Graphs in (C)–(E) show mean ± SD; gray data points represent independent experiments; n, number of egg chambers scored; Fisher’s exact test (ns, not significant).
(F and H) Time-lapse images of the follicular epithelium (surface views) expressing LARIAT, GFP::aPKC, and H2A::RFP (chromatin) and stained with membrane marker. Bin-2 (F) or Colch (H) was added at least 15 min prior to clustering from min 0 onward. Epithelial gaps (arrows) form adjacent to dividing cells (colored) despite cytokinesis failure (F, chromatin decondenses without chromosome separation) or mitotic arrest (H, condensed chromatin throughout the video).
(G and I) Epithelial-gap origin according to cell division stage of neighboring cells in LARIAT egg chambers treated with Bin-2 (G) or Colch (I); n, number of gaps scored; scale bars, 10 μm.