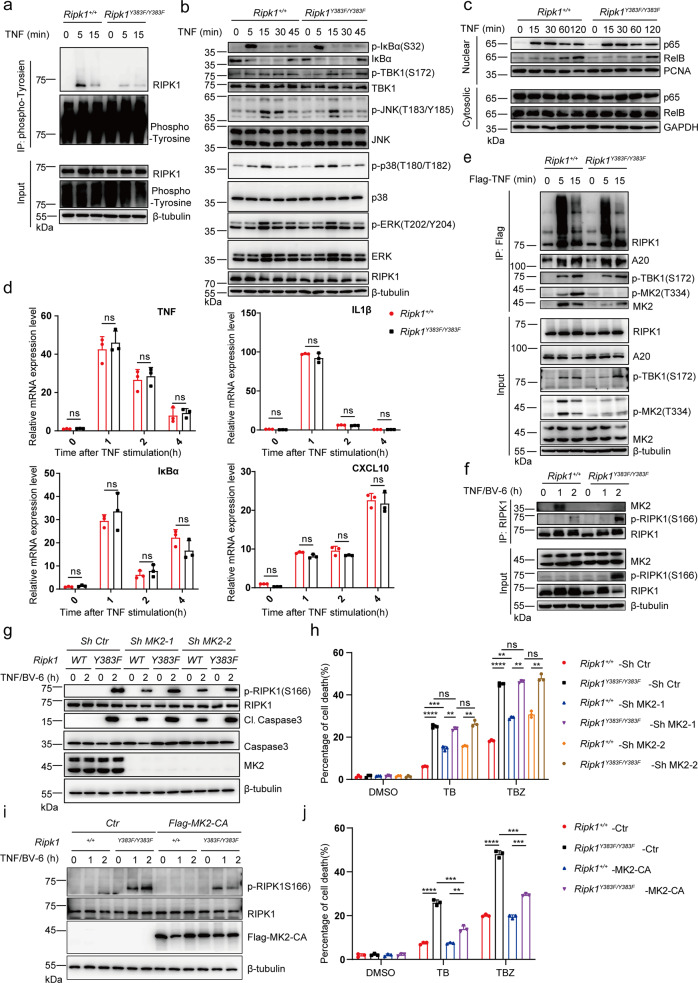
Fig. 4. Tyrosine phosphorylation of RIPK1 is essential for limiting RIPK1 kinase activity.
a Primary Ripk1+/+ and Ripk1Y383F/Y383F BMDMs were stimulated with TNF for indicated time points. Whole-cell lysates were immunoprecipitated with anti-phospho-tyrosine antibody for western blotting with indicated antibodies. b, c Ripk1+/+ and Ripk1Y383F/Y383F immortalized MEFs were stimulated by TNF (10 ng/ml) for indicated time points. Cytosolic (b) and nuclear (c) extractions were collected for western blotting with indicated antibodies. d Primary Ripk1+/+ and Ripk1Y383F/Y383F BMDMs were stimulated with TNF at different time point. The transcriptional and expression level of inflammatory NF-κB target genes were measured by qPCR. Ripk1+/+ and Ripk1Y383F/Y383F immortalized MEFs were stimulated by Flag-TNF (100 ng/ml) (e) or TNF (10 ng/ml) /BV-6 (2.5 μM) (f) for indicated time points. Whole cell lysates were immunoprecipitated by anti-Flag resin (e) or anti-RIPK1 antibodies (f) for western blotting with indicated antibodies. Ripk1+/+ and Ripk1Y383F/Y383F immortalized MEFs with or without knockdown of MK2 (g, h) or overexpression of constitutively active MK2 (MK2-CA) (i, j) were treated with TNF (10 ng/ml) /BV-6 (2.5 μM) for indicated time points. Whole-cell lysates were collected for western blotting (g, i). Cell death was measured by SytoxGreen positivity (h, j). T: TNF (10 ng/ml); B: BV-6 (2.5 μM); Z: zVAD.fmk (20 μM). In d, h, j, data are represented as mean ± SEM (n = 3 independent cell samples). Statistical significance was determined using a two-tailed unpaired t test. n.s. p > 0.05; ** p < 0.01; ***p < 0.001; ****p < 0.0001. Source data are provided with this paper.