Figure 2.
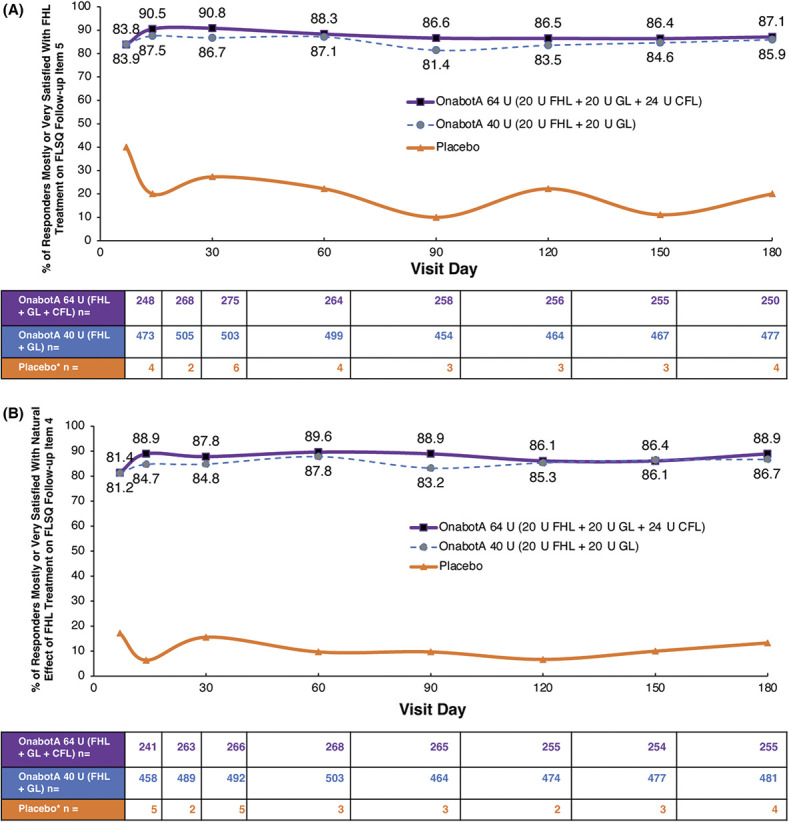
Satisfaction with treatment for FHL. (A) Satisfaction with effect of onabotA treatment on FHL: FLSQ follow-up Item 5 (overall satisfaction with treatment). (B) Satisfaction with the natural effect of onabotA treatment on FHL: FLSQ follow-up Item 4 (satisfaction with receiving a natural look). Responders were patients who received onabotA and had investigator-assessed ≥1-grade improvement from baseline in FHL severity on the FWS at Day 30 (pooled 142/143 studies). *Fewer than 7 patients in the placebo group had a reported ≥1-grade improvement from BL in FHL severity on the FWS at Day 30. BL, baseline; CFL, crow's feet lines; FHL, forehead lines; FLSQ, Facial Line Satisfaction Questionnaire; FWS, Facial Wrinkle Scale; GL, glabellar lines; onabotA, onabotulinumtoxinA.
