Abstract
Traditional nanoparticle carriers such as liposomes, micelles, and polymeric vehicles improve drug delivery by protecting, stabilizing, and increasing the circulatory half-life of the encapsulated drugs. However, traditional drug delivery systems frequently suffer from poor drug loading and require an excess of carrier materials. This carrier material excess poses an additional systemic burden through accumulation if not degradable, the need for metabolism, and potential toxicity. To address these shortcomings, minimal-carrier nanoparticle systems and pharmacoactive carrier materials have been developed. Both solutions provide drug delivery systems in which the majority of the nanoparticle is pharmacologically active. While minimal-carrier and pharmacoactive drug delivery systems can improve drug loading, they can also suffer from poor stability. Here, we review minimal-carrier and pharmacoactive delivery systems, discuss ongoing challenges and outline opportunities to translate minimal-carrier and pharmacoactive drug delivery systems into the clinic.
Keywords: cancer, bioactive materials, protein nanoparticle, nucleic acids, drug self-delivery, pharmacokinetics, carrier-free
Graphical Abstract
1. Minimal-carrier drug delivery systems and pharmacoactive drug carriers
The delivery of small molecule drugs, proteins, and nucleic acids is often hampered by their poor pharmacokinetics and insufficient stability in the presence of degradative enzymes [1,2]. In the case of small molecules, a lack of specific disease targeting can cause non-specific interactions and systemic off-target adverse effects in healthy tissues [3]. Proteins and nucleic acids often require a delivery vehicle to maintain their structure long enough to reach their target cells and site of action [4–6].
Over the last three decades, nanoparticles have been increasingly used to enhance drug delivery outcomes for both small molecule drugs and biologics, specifically nucleic acids [7]. Nanoparticles are attractive as they can significantly enhance the circulatory half-life of small molecules, proteins, and nucleic acids, and their formulation can be adjusted to modulate size, surface charge, composition, and other factors [8–15]. Nanoparticles can also be designed to provide sustained release of small molecules [16–19]. Thus, by improving the pharmacokinetics of therapeutics, providing drug protection, and enhancing accumulation at disease sites, nanoparticle-based therapies have improved clinical outcomes [20].
In 1995, the FDA approved the first nanotherapeutic drug, liposomal doxorubicin aka Doxil®. Liposomal doxorubicin is more effectively delivered to tumors with a more favorable safety profile compared unencapsulated free doxorubicin [21]. Doxil® also presents the first FDA-approved nanoparticle to use PEGylation to increase circulation time. In 1997, Optison™ (Perflutren Protein-Type A Microspheres) was FDA-approved and introduced the idea of using a carrier material that is native to the body, i.e., human albumin protein, to deliver therapeutics [22]. In 2005, Abraxane® was approved, which also uses albumin as a carrier to form nanoparticles and to more effectively deliver paclitaxel to tumors [23]. Another significant advancement was introduced in 2017 when VYXEOS® was approved as the first liposomal formulation delivering two drugs in a synergistic drug ratio to achieve enhanced efficacy (daunorubicin and cytarabine = 1: 5 molar ratio) [24]. This would be difficult to achieve without a nano-drug delivery system. As of 2021, there are over 25 approved nanotherapeutics, and there have been at least 75 new nano-therapeutic clinical trials since 2016 [25]. Among all FDA-approved nanoparticle therapies, approximately 39% were for cancer, 35% were for iron replacement therapy (Injectafer® and FERAHEME®), and 26% were for other applications, e.g., imaging agents (DEFINITY® and Optison™), and fungal treatments (AmBisome®), [25]. Between 2016 and 2021, three new nano-therapeutics were approved by the FDA, two of which were cancer therapies (VYXEOS® and Hensify®). More recently, advanced nano-drug delivery systems such as lipid nanoparticles (LNP) have led to approvals of first-in-class RNA-based therapeutics. For example, the first RNA-based therapeutics Onpattro® (approved in 2018), delivers siRNA to hepatocytes [42]. Since the COVID-19 pandemic, LNP-based nano-drug delivery systems have gained substantial research momentum. This is due to the transformative success of two novel LNP-enabling mRNAbased vaccines demonstrated by Moderna and Pfizer-BioNTech [43].
While existing clinically approved nano-therapeutics provide additional pharmacokinetic & therapeutic benefits over small molecules, naked nucleic acids, and other therapeutics, they also have their limitations, such as the limited drug loading capacity [26,27], which means that most of the nanoparticle’s content is inactive carrier materials (excipients) instead of active therapeutic cargo. To overcome this limitation, strategies to achieve high drug loading nanotherapeutics have been comprehensively described by Shen et al., Wang et al., and Liu et al. [28–30]. As the carriers need to be degraded and/or metabolized, high excipient use could still lead to adverse reactions, inflammation and induce immune responses [31–37]. This is especially a concern for slowly or non-degradable polymeric or non-degradable metallic- and inorganic carriers, which can accumulate in organs and tissues [38]. Due to limited drug loading, administration of a larger quantity formulation is required to attain an effective drug dose, which in turn can exacerbate adverse events. These issues in part contribute to the challenges in successfully translating nano-based therapies into the clinic. Of the nano-therapeutics that have reached phase three clinical trials, only 14% were successful and led to FDA-approved therapies [39,40]. Thus, there is a need to develop new drug delivery systems with minimal carrier materials to maximize the therapeutic potential and to improve biocompatibility. Similarly, there is great interest in developing carrier materials with inherent pharmacological activity or bioactivity.
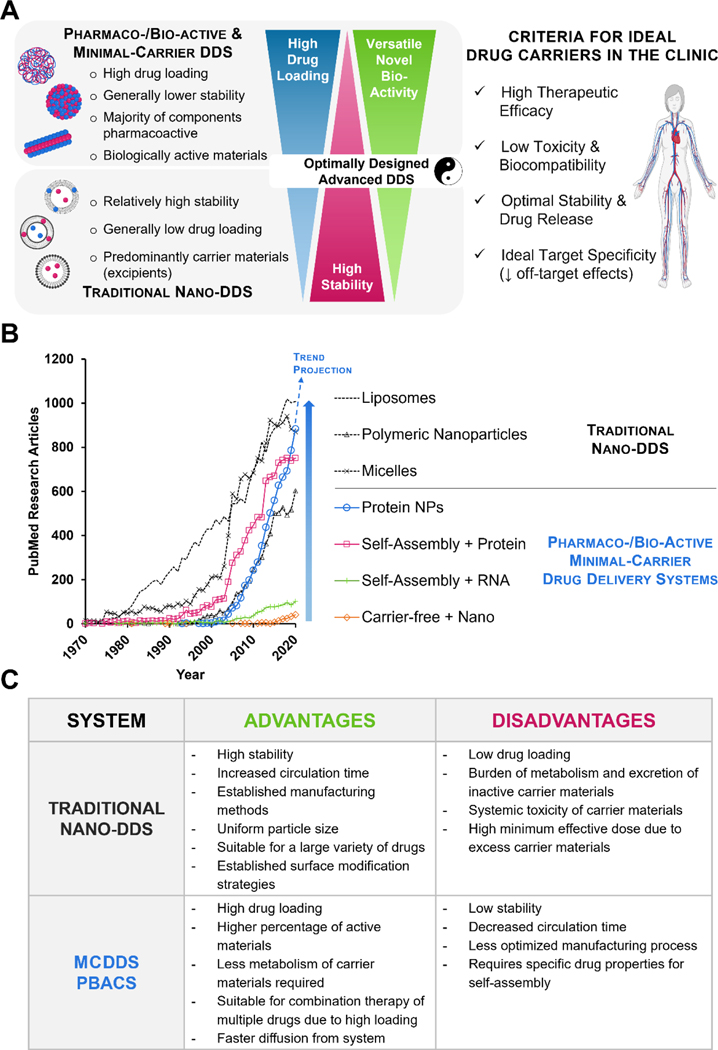
In this review, we discuss two emerging drug delivery systems: i) delivery systems (MCDDS) designed to minimize carrier materials to reduce overall toxicity and ii) pharmacoactive or bioactive carrier systems (PBACS), in which the carrier materials are used to improve targeting, pharmacokinetics, and display inherent pharmacological activity and/or bioactivity [41]. A comparison of traditional DDS, MCDDS, and PBACS is shown in Figure 1. While traditional drug delivery systems including liposomes, micelles, polymeric nanoparticles, and polymersomes continue to dominate the field, an increasing number of publications have reported the use of MCDDS and PBACS since 2000 (Figure 1B). As drug delivery approaches continue to progress, the benefits of a bioactive or pharmacoactive carrier system over an inactive carrier system are gradually being appreciated and represent an exciting area of innovation.
Figure 1. Emerging nanosized minimal-carrier and pharmacoactive drug delivery systems compared to traditional drug delivery systems.
(A) Comparisons between traditional nano-DDS and MCDDS/PBACS highlighting the contrasting and complementary features of both systems. Optimized combinatorial systems could lead to ideal drug carrier candidates for clinical translation. (B) The emerging trend of MCDDS and PBACS, especially protein therapeutics, is fast approaching the gold-standard polymeric drug delivery systems in recent years. *PubMed search terms: Liposome, (liposome[Title/Abstract]) NOT (review[Publication Type]); Polymeric nanoparticles, (Polymeric nanoparticle[Title/Abstract]) OR ((PLGA[Title/Abstract] AND (drug[title/abstract])) NOT (review[Publication Type]); Micelles, (Micelle[title/abstract]) NOT (review[Publication Type]); Protein NPs, (protein[Title/Abstract]) AND (nanoparticle[Title/Abstract]) NOT (review[Publication Type]) NOT (albumin[Title/Abstract]); Self-Assembly + Protein, (self-assembl*[Title/Abstract]) AND (protein[Title/Abstract]) NOT (review[Publication Type]); Self-Assembly + RNA, (self-assembl*[Title/Abstract]) AND (RNA[Title/Abstract]) NOT (review[Publication Type]); Carrier-free + Nano, (Carrier-free[Title/Abstract]) AND (nano*[Title/Abstract]) NOT (review[Publication Type]). (C) Comparison of traditional nano-DDS and MCDDS.
2. Improved therapeutic efficacy with high drug-loading minimal-carrier delivery systems
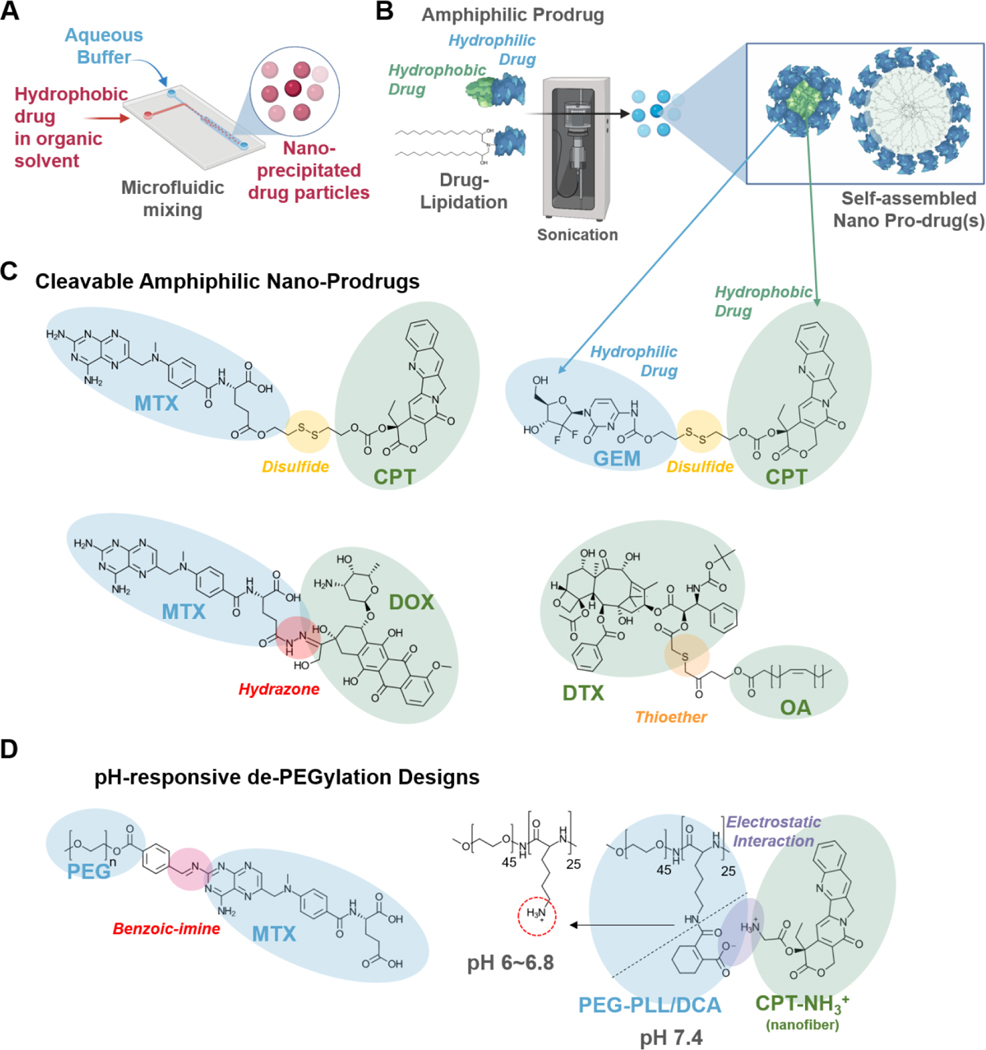
MCDDS are therapeutics that use minimal drug carrier materials and where most constituents are the drug (Figure 1, Table 1). MCDDS could have a maximum loading capacity of up to 100% where no or minimal amounts of carriers are used for the formation of nanoparticles, microparticles, fibers, or other delivery systems. In these formulations, drugs self-assemble into nanoparticles or microparticles by precipitation, solvent exchange, or self-assembly (Figure 2A) [44–47].
Table 1.
Summary of Minimal-Carrier Drug Delivery Systems.
| Name | API | Fabrication | Size (nm) | API Release | Indication | Efficacy | API Loading (w/w%) | Ref |
|---|---|---|---|---|---|---|---|---|
| DOX NPs † | DOX | Solvent Exchange |
60 | Diffusion | 4T1 tumor | ↑ 2.6X tumor inhibition | 90 | [44] |
| HCPT NCs | HCPT | Precipitation + homogenization | 130 | Diffusion | 4T1 tumor | ↑ 10X uptake ↑ 2X tumor inhibition |
75 | [45] |
| PTX NRs | PTX | Solvent Exchange |
500 ×40 |
Diffusion | KB cells | ↑ 10X cytotoxicity | 93 | [46] |
| CPT-PEG NFs | CPT | Self-assembly + PEG coating | 1000× 100 | pH | 4T1 SKOV-3, OVCAR-3, MDA- MB-468, MDA-MB- 231 cells |
↑ 5X blood T1/2 | 85 | [47] |
|
CPT-SS-
GEM NPs |
CPT, GEM |
Self-assembly with disulfide bond | 100–200 | Redox | A549, NCI-H460, HCT116, HT-29, MCF-7/ADR |
↑ 2X uptake | 42 CPT 32 GEM |
[48] |
| GEM-CPT NPs | GT, CPT |
Self-assembly with disulfide bond | 41 | Redox | HeLa, MCF-7 cells | ↑ 6X release | 75 | [49] |
| DTX-OA NPs | DTX, OA |
Self-assembly with thioether linker | 153 | Redox | PC3, MCF-7 cells | ↑ 5X release | 57.8 | [50] |
|
MTX-HCPT
NPs |
MTX, HCPT | Co-assembly | 160 | pH/redox | HeLa MCF-7, A549, NIH-3 T3 cells |
↑ 3X uptake ↑ 1.25X drug accumulation |
91.2 | [51] |
|
MTX-DOX
MTX-CPT NPs |
MTX, DOX, CPT |
Self-assembly with disulfide linker | 80 (CPT) 39 (DOX) |
pH/redox | MCF-7 tumor | ↑ 2.7X tumor inhibition | N/A | [52] |
|
HCPT-
DOX NPs |
HCPT , DOX |
Co-assembly | 232 | Diffusion | MCF-7 tumor | ↑ 1.6X nuclear accumulation | N/A | [53] |
|
H
2
TPPS-
DOX NPs |
DOX | Self-assembly | 90 | PDT | Anti-MCF-7/ADR cells | ↑ 2.6X drug release | 42 | [54] |
| SN38-Ce6 NPs | SN38 | Antisolvent precipitation | 150 | PDT | 4T1 tumor | ↑ 1.3–4.5X tumor inhibition | N/A | [55] |
|
CPT-GEM-
AIEgens NSs |
CPT, GEM |
Self-assembly | 150 | NIR | A549 cells | ↑ 2.5X tumor inhibition | N/A | [56] |
|
DOX-
NH4CO3- PDA NPs |
DOX | Co-assembly with PDA coating | 70 | NIR | HeLa cells | ↑ 1.7X photothermal effect |
85 | [57] |
| Ir/Cb NPs | Ir and Cb |
Self-assembly with ester linker | 88 | pH/hydro lysis | MCF-7 tumor | ↑ 20X blood retention, +1.8X tumor inhibition | N/A | [58] |
| MTX-CPT NPs | MTX, CPT | Self-assembly with ester linker | N/A | pH/hydro lysis | N/A | N/A | N/A | [59] |
NPs: nanoparticles; NRs: nanorods; NCs: nanocrystals; NFs: nanofibers; NSs: nanospheres
Figure 2. Formulation and synthetic approaches for high drug-loading MCDDS.
A) Microfluidic systems to rapidly and controllably precipitate hydrophobic drugs into drug nanoparticles in aqueous media. B) Amphiphilic self-assembly using hydrophilic-hydrophobic dual-drug conjugates or lipidation of hydrophilic drugs to form nano-prodrugs. C) Cleavable amphiphilic nano-prodrugs require a stimuli-responsive drug release mechanism to disassemble the nanocarrier and release the cargo. Such mechanisms include the use of redox and pH-sensitive disulfide, thioester, and hydrazone bonds. D) PEGylation is beneficial for nano-therapeutics, especially when extended blood circulation is required. However, PEGylation also hinders the cellular uptake of nanotherapeutics; thus, having pH-responsive de-PEGylation mechanisms could be advantageous to further improve the target specificity and efficacy of MCDDS. Such designs include acid liable benzoin-imine linkages [60], as well as cleavable DCA-capped PLL-b-PEG interactions [61].
The first example of MCDDS designs is pure drug nanoparticles. Pure drug nanoparticles can improve delivery outcomes compared to free drugs by increasing the circulatory half-life. For example, doxorubicin (DOX), a common chemotherapeutic drug, could be formulated into spherical nanoparticles using a solvent exchange method by precipitating the hydrophobic free-base DOX molecules in an aqueous solution [44]. The DOX nanoprecipitates were then PEGylated to increase blood circulation time. These DOX nanoprecipitates showed an extremely high drug loading capacity (90.47% w/w) and 2.58-fold greater tumor inhibition than free DOX (hydrophilic DOX HCl salt). When pure 10-hydroxycamptothecin (10-HCPT) nanocrystals were formulated at 75% (w/w) drug loading using microprecipitation and high-pressure homogenization, they had 10-fold greater cellular uptake and exhibited a 2-fold increase in anti-tumor activity than existing injections of free 10-HCPT at the same dose [45].
Pure drugs can also be assembled into nanorods, which show increased cellular uptake compared to spherical particles [10]. Li et al. found that paclitaxel (PTX) molecules can be formulated into nanorods with 93% drug loading and delivery efficiency ten times greater than of the free drug [46]. These PTX rods were prepared using anti-solvent precipitation and were functionalized with PEG and folic acid for increased circulation and tumor cell targeting. Camptothecin (CPT) was formed into nanofibers with an amide bond using self-assembly and functionalized with a pH-responsive PEG coating [47]. These fibers were 85% pure CPT and increased the drug half-life five-fold compared to free CPT.
Other approaches to engineer MCDDS include the synthesis of amphiphilic prodrug conjugates that self-assemble into nanoparticles (Figure 2B) [48–51]. Through this approach, two or more pure drugs can be combined into a MCDDS with high drug loading to improve efficacy and to avoid multi-drug resistance. These can be formed through co-assembly methods or self-assembly methods, with linkers that utilize thiol or ester interactions. For example, CPT was functionalized with a disulfide linkage to form nanoparticles with gemcitabine (GEM) through self-assembly, as CPT is hydrophobic and GEM is hydrophilic. This system showed good drug loading (42.6% w/w CPT and 32.2% w/w GEM), high dissociation in tumor-mimicking environments (90%), and a 1.5–2.5 fold increase in cellular internalization versus free drug [48]. Hou et al. formed another self-assembled nanoparticle of CPT and GEM using a disulfide bond linker [49]. This system showed high drug loading (~75%) and improved cytotoxicity against two cancer cell types with the two drugs combined compared to the individual drugs. Jing et al. demonstrated a different minimal-carrier approach by synthesizing a docetaxel (DTX) prodrug with thioether-conjugated oleic acid (OA). The DTX-OA prodrug self-assembled into spherical nanoparticles with high drug loading at 57.8% (w/w) that was further PEGylated to improve blood circulation [50]. Other nano-prodrugs designs include tunable MTX & HCPT self-assembled nanospheres with drug loadings at 79% and 21% (w/w), respectively [51].
3. Triggerable MCDDS improve therapeutic specificity and efficacy
Drug release from MCDDS could also be triggerable and react to changes in the environment, such as the pH or redox state inside diseased tissues (Figure 2C) [48,50,52,53]. To accomplish this, Hou et al. conjugated methotrexate (MTX), a hydrophilic drug, to the hydrophobic drugs DOX and CPT using pH-sensitive hydrazone bond and disulfide bond linkers, respectively, resulting in self-assembling amphiphilic nanoparticles [52]. MTX was used as both an anticancer drug and a targeting agent that increased cell internalization due to its folic acid-mimicking structure. These two minimal-carrier nanoparticles were successfully internalized into tumor cells, released their drug cargo once exposed to the tumor environment, and reduced tumor volume by around 2.7-fold when used together.
Another study demonstrated the use of disulfide bonds as a redox-sensitive linker for minimal-carrier nanoparticles by conjugating CPT and GEM as described earlier [48]. This demonstrates the ability of these nanoparticles to not only show improved efficacy compared to free drugs but also increase drug efficacy by producing synergistic effects of the combined drugs. A thioether bond can also be used as a redox-responsive drug nanoparticle linker. For example, Jing et al. used a thioether linker to form spherical nanoparticles of docetaxel and oleic acid [50]. The redox-sensitive drug release mechanism reduced general toxicity while maintaining efficacy when compared to free DTX. Patients sometimes require both DOX and 10-hydroxycamptothecin (10-HCPT), but they cannot be dosed simultaneously due to their stored pH differences, which causes acid-base neutralization and precipitation upon mixing [53]. When formed into self-assembled nanoparticles, however, these two drugs showed a ~1.6-fold increase in accumulation in the nuclei that led to increased cytotoxicity of breast cancer cells compared to the drugs delivered freely. Thus, minimal-carrier nanoparticles can solve solubility issues and poor interactions when delivering multiple drugs without introducing the additional burden of carrier materials.
In addition to responsive prodrug linkers, pH-sensitive PEG coatings can also be used to form smart MCDDS (Figure 2D). One study combined MTX with HCPT using the same small molecule amphiphilic interactions for self-assembly, then coated the nanoparticle with PEG [51]. The MTX-HCPT nanospheres contained a pH-responsive PEG layer by post-insertion of PEG-conjugated methotrexate (MTX) using a benzoic-imine linkage. When the particle entered the acidic tumor microenvironment, the pH-responsive PEG coating detached from the system and allowed the release of the small molecule drugs. This increased tumor cell uptake and drug accumulation compared to non-pH responsive control particles. PEG can also be used as a pH-responsive coating for pure drug nanorods. CPT nanofibers functionalized with a pH-responsive PEG coating could deliver the positively-charged nanofibers to tumor sites and enter cell membranes while minimizing interactions with cells in healthy tissues [47].
In addition to physicochemical sensing mechanisms, MCDDS can also be triggered through external energy. Photodynamic therapy (PDT) uses photosensitizers that, when exposed to light, produce reactive oxygen species (ROS) that can react with nearby molecules and induce tumor cell death. For example, Liu et al. used a water-soluble photosensitizer (H2TPPS) and doxorubicin molecules to form self-assembling nanospheres that activated upon light irradiation. This combination of redox-responsive and photo-responsive therapy-induced apoptosis upon light exposure to counteract drug resistance of doxorubicin [54]. Another study combined chemotherapy and PDT using SN38, a chemotherapeutic, and Ce6, a photodynamic therapy agent, using antisolvent precipitation [55]. Both molecules are insoluble and suffer from poor delivery outcomes, but when combined into nanoparticles, their tumor accumulation was improved 12-fold.
Zhang et al. used CPT-ss-GEM nanowires to form nanoparticles with aggregation-induced emission luminogens (AIEgens), which are compounds activated by near-infrared (NIR) light [56]. These nanoparticles were formulated by co-assembly to form a redox-responsive system capable of both imaging and geometry modification using NIR. Nanowires exposed to AIEgens organized into spherical structures. Once delivered, the redox-responsive nanoparticles did not release their cargo until they arrived at the tumor site, while NIR emissions from the AIEgens could be used for imaging. Another study added NIR activation to minimal-carrier DOX nanoparticles by co-assembly of DOX molecules and NH4HCO3, which releases CO2 and NH2 after thermal energy is added [57]. These nanoparticles were coated in a polydopamine film that was broken down by CO2 and NH2, effectively protecting the drug cargo until activation by NIR irradiation. This system had high drug loading (85.8%) and showed both increased tumor accumulation and reduced tumor growth compared to chemotherapy or photothermal therapies alone.
4. Stability considerations with minimal-carrier drug delivery systems
Since MCDDS might not always contain a stable carrier shell, they could suffer from undesired stability and premature disassembly before reaching the disease site. One common method employed to improve stability is adding PEG to the nanoparticle to provide steric shielding and to increase circulatory half-life [62]. For example, Yu et al. added PEGylation to pure DOX nanoparticles and, in doing so, increased the circulatory half-life 18-fold compared to pure DOX drugs [44]. Li et al. used PEG with folic acid to increase the circulation time and targeting of pure PTX nanoparticles [46].
Linkers have also been used to improve MCDDS stability by adding internal structural support to the nanoparticles, such that the self-assembled structures remain stable in blood circulation for longer. These can be engineered to release the drug from the MCDDS when preferred redox conditions are met at tumor sites. Huang et al. combined two anticancer drugs, irinotecan (Ir) and chlorambucil (Cb), using a specifically designed hydrolyzable ester linker that breaks in the tumor microenvironment [58]. These drugs formed 75 nm nanoparticles in water due to their opposite polarities and were released in tumor tissues by breaking the linker bond. The bloodstream retention after 12 hours was 20-fold higher than free Ir and Cb, demonstrating the improved pharmacokinetics of the MCDDS compared to the free drug. Another group compared CPT and MTX self-assembled nanoparticles using an ester linker versus free drug self-assembled nanorods and found that the ester linkage was more stable [59]. Additionally, the ester linkage allows for pH activation, which protects healthy cells from the effects of chemotherapeutic drugs.
Various geometrical designs have also been utilized to increase the stability of MCDDS. Forming components into spherical or rod-shaped nanoparticles increases stability compared to drug formulations not organized into specific structures [46,47].
Such approaches could also be adopted using pharmacoactive or bioactive drug carrier materials. For example, DNA nanostructures have been used to improve the stability of biologic-based carrier materials and are discussed in more detail below. They can be formed into complex triangular and nanoring structures that show increased stability compared to unorganized structures [63,64].
5. Enhancing drug efficacy through pharmacoactive or bioactive carrier materials
Pharmacoactive or bioactive drug carriers (PBACS) are drug carriers with inherent pharmacological or biological activity (Table 2). PBACS could significantly improve the therapeutic efficacy of the drug cargo by acting on the system in combination with the active drug without the need for excessive, inactive carrier materials. Emerging PBACS can be roughly organized into the following categories: i) biological materials only, ii) small molecule (drug) scaffolded biological materials, iii) nucleic acid scaffolded carriers, and iv) others (such as vitamins and sugar), as described below.
Table 2.
Summary of pharmacoactive and bioactive carrier systems (PBACS)
| Name | API | Active Carrier | Carrier Activity | Size (nm) | Indication | Efficacy | API Loading (w/w%) |
Ref |
|---|---|---|---|---|---|---|---|---|
| DHFR-bisMTX CSANs | DHFR2a ntiCD3 | bisMTX | CD3+ trafficking | 280–494 | CD3+ T- leukemia cells |
↑ 13X cytotoxicity | N/A | [63] |
| RNAi-DOX-DNA | DOX | DNA, RNAi, aptamer |
Anti-MDR gene therapy | N/A | MCF-7R tumor | ↑ 4 tumor inhibition | N/A | [64] |
| CXCR4-scFv-RBM | miRNA | CXCR4-scFv | Macrophage polarization | 113–119 | 4T1 tumor | ↑ 2X tumor inhibition | 90 | [65] |
| HA-PTX-Caspase 3 | PTX | Caspase 3 and HA | Apoptosis | 160 | MCF-7 | ↑ 1.5X accumulation, ↑ 7X caspase 3 |
N/A | [66] |
| siRNA-Aptamer NPrs | Rab26 siRNA | MUC-1 | Apoptosis | 14–21 | A549, H1299 cells |
↑ 9.1X cell internalization | N/A | [67] |
| DNA NTs | DNA | DNA | miRNA knockdown | 22.4 | H1299, MCF-7 | ↑ 15X cellular uptake | N/A | [68] |
| RNA micelles | PTX | RNA | Apoptosis | 104–133 | KB cells | ↑ 8.1X cancer cell apoptosis | N/A | [69] |
| DOX-siRNA NA | DOX | siRNA | ICD | 25–45 | CT26 cells | ↑ 0.48X tumor inhibition | 21.67 | [70] |
| UA-DOX NPs | DOX | UA, Aptamer | Anti-metastasis | 108.9 | BT474 cells | ↑ 2X drug retention | 99.6 | [71] |
| DOX-Aptamer | DOX | Aptamer | Antiproliferation | 9- 11.6 |
Anti-PC3, 4T1 cells | ↑ 1.99X tumor inhibition | N/A | [72] |
| VES-Ir NPs | Ir | VES | Apoptosis | 75.4 | MCF-7, A549 | ↑ 1.86X tumor inhibition | 100 | [73] |
| MTX-MAN NPs | MTX | Mannose | Apoptosis | 100 | MCF-7 | ↑ 1.4X tumor inhibition | N/A | [74] |
NPrs: nanoprisms; NTs: nanotubes; NA: nanoassembly; NPs: nanoparticles
In the first category, our group previously developed a pure biological-PBAC composed of a CXCR4 single-chain variable fragment (scFv) antibody fused to protamine, an RNA-binding motif (RBM). When mixed with a small RNA, such as a miRNA, the CXCR4-scFv-RBM fusion protein formed nanoplexes [65]. Here, the scFv not only mediated binding to the chemokine receptor CXCR4 (thus enhanced cargo delivery), but also acted as a pharmacological agent by polarizing macrophages to the tumor-suppressive M1 phenotype via CXCR4 blockade (as an immune-modulation carrier). Furthermore, the CXCR4-scFv fused to an RNA-binding peptide served as a carrier and could deliver miRNA to macrophages to inhibit tumor growth (Figure 3A).
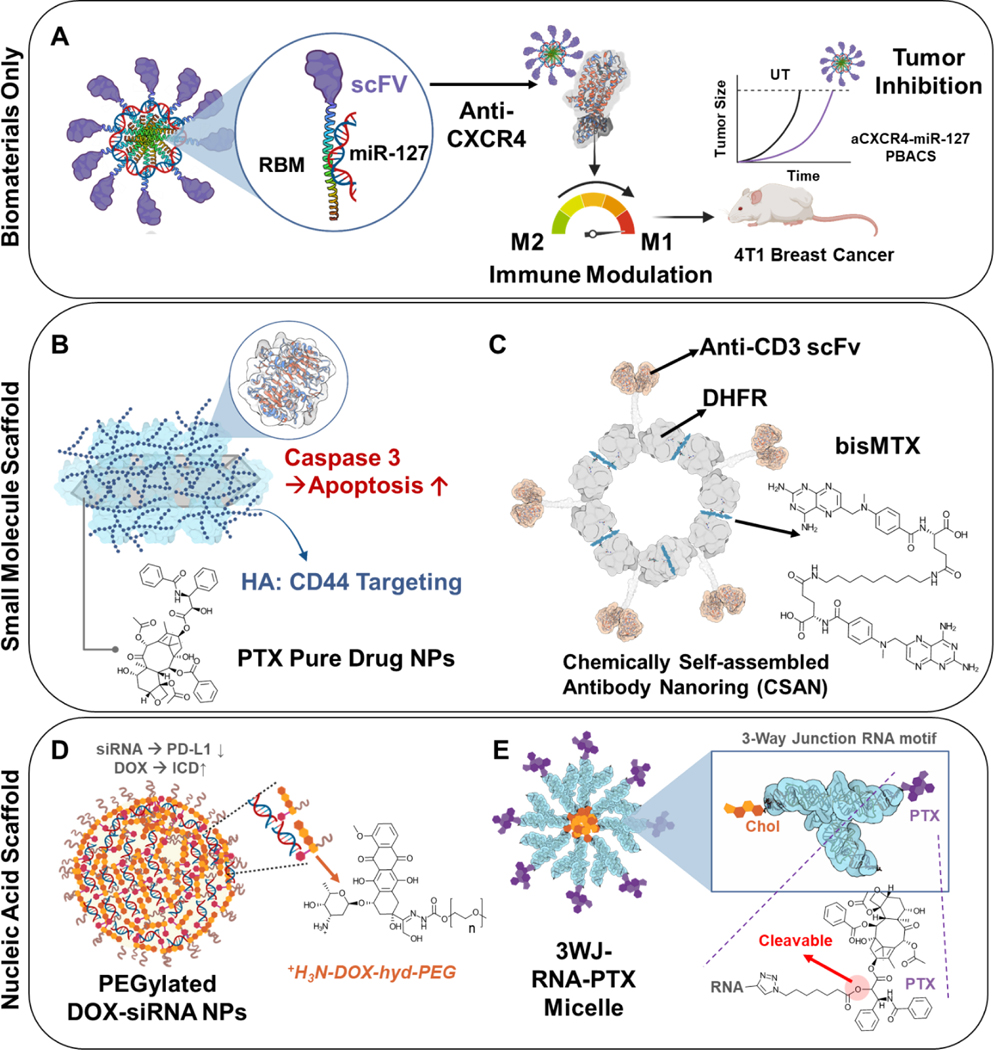
Figure 3. Different types of pharmaco- and bioactive carrier systems.
(A) Biomaterials such as peptides can be used as active carrier materials to transport cargo. In this example, both an RNA binding motif peptide and scFv protein are used to deliver miRNA [65]. This CXCR4-targeting therapeutic was able to modulate immune function and inhibit tumor growth simultaneously. (B) Small molecule drugs can be formed into scaffolds to deliver additional cargo to enhance the system’s efficacy. PTX nanorods were constructed into a scaffold able to carry caspase three enzymes, and an HA coating was added for additional function [66]. (C) bisMTX can be used as an active scaffold formed into a ring structure that can carry fusion scFv-DHFR peptides while being a chemotherapeutic drug [63]. (D) Lastly, nucleic acids can be formed into complex architectures able to load various cargo. siRNA was engineered into nanoparticles able to carry DOX molecules to initiate ICD [70]. (E) RNA can be conjugated to PTX molecules to form a cleavable nanoparticle. There is potential for the RNA carrier to be therapeutic depending on the RNA sequence used [69].
The second category of PBACS is constructed by using pharmacologically active small molecules as the carrier scaffold. For example, caspase 3, an enzyme important for apoptosis, was absorbed onto paclitaxel (PTX) pure-drug nanorods followed by hyaluronic acid (HA) coating to target the CD44 receptor [66]. This functionalized pure-drug nanorod could bypass endo-lysosomes, leading to a 1.5-fold increase in tumor accumulation and 100% tumor growth inhibition (Figure 3B). Antibodies can also be engineered to organize into chemically self-assembled antibody nanoring (CSAN) structures to deliver small molecular anticancer drugs with improved targeting and pharmacokinetics. Fegan et al. formulated CSANs using dihydrofolate reductase (DHFR) anti-CD3 scFv fusion proteins and bis-methotrexate (bisMTX) [63]. This PBACS could internalize into cells and cause CD3+ T-leukemia cell trafficking. The CSANs were also able to include additional binding ligands around the nanoring to capture fluorophores for labeling. This system was rapidly internalized by CD3+ cells in vitro and remained stable for several hours until disassembly with trimethoprim (Figure 3C).
The third category of PBACS uses nucleic acids as scaffolds. Nucleic acids are another option to consider when designing PBACS since they are native to the body and can produce various pharmacological effects, including regulating gene expression. Nucleic acids are being explored as bioactive carriers due to their ease of assembly and ability to induce biological effects. Both DNA and RNA can be formed into various nanostructures such as prisms, nanorods, and micelles [67–69]. Chen et al. designed a nanoparticle using π-π stacking and electrostatic interactions to combine siRNA, doxorubicin, and PEG into a drug carrier system [70]. The final nanoparticle contained around 4% siRNA and 22% DOX loading. This system counteracted the immunosuppressive genes upregulated in the presence of doxorubicin using siRNA as the active material capable of suppressing PD-L1 (programmed death-ligand 1) expression, which increased tumor internalization while increasing the therapeutic efficacy of the carried DOX (Figure 3D). Aptamers show similar targeting and complex structure organization capabilities that make them good candidates for pharmacoactive carrier materials. Jiang et al. developed a minimal-carrier nanoparticle composed of ursolic acid and DOX with a HER2 aptamer conjugated to its surface via electrostatic interactions [71]. The HER2 aptamer was used to target cancer cells as well as increase drug retention inside HER2-overexpressing cells. They found that drug retention increased 2-fold in these cancer cells, and the system significantly inhibited tumor growth without additional side effects. Another study by Taghdisi et al. formed cancer cells targeting aptamer nanostructures that delivered DOX using a “three-way junction pocket” design [72]. Three aptamer strands were combined to form a triangular nanostructure with DOX molecules contained throughout and PEG attached at the three vertices for improved pharmacokinetics. These pharmacoactive carrier aptamers induced a form of non-apoptotic cell death through nucleolin binding. This system increased tumor growth inhibition compared to free doxorubicin with significantly lower cytotoxicity to healthy cells than cancer cells (Figure 3E).
A new avenue to explore in the field of active protein-based and nucleic acid-based carrier materials is peptide nucleic acids (PNA). PNA is a biopolymer made of a neutrally-charged peptide backbone that can bind nucleic acids through Watson-Crick-Franklin base pairing [75]. It can be used as a carrier for both peptides and nucleic acid-based therapeutics while being active itself. It can also be engineered into self-assembling nanoparticles or micelles. Therapeutically active nucleic acids have also been organized into micelles that can carry active drugs while inducing biological effects themselves to improve treatment outcomes. Shu et al. formed RNA micelles by conjugating RNA to a lipid that self-assembled into RNA micelles [69]. Paclitaxel was successfully loaded into these micelles to improve cancer cell internalization of the drug and reduced cancer proliferation. In this specific case, the RNA molecules served as both the micellar carrier to deliver paclitaxel and as a pharmacological agent to induce apoptosis in cancer cells, thus allowing the system to deploy multiple anticancer mechanisms simultaneously.
Finally, aside from antibodies, peptides, and nucleic acids, other pharmacologically active carrier materials and bioactive materials, such as vitamins and sugars, have also been briefly explored as therapeutic carrier materials. For example, Vitamin E succinate has been shown to have anticancer cell properties and enhance the effects of other anticancer drugs. One study combined vitamin E succinate with irinotecan into a 75.4 nm 1:1 drug ratio nanoparticle using an esterification reaction [73]. They found that this system suppressed MCF-7 and A549 cancer cells more than drugs delivered freely. Sugar such as mannose could also be formulated into a nanosized drug carrier that can avoid macrophage uptake and increase the efficacy of the delivered drug. Fan et al. designed a self-assembling nanoparticle system of methotrexate, an anticancer drug, with mannose through a hydrolyzable ester bond for stimuli-responsive capabilities [74]. The results showed that this system effectively decreased toxicity to healthy cells and that mannose effectively enhanced the anti-tumor activity of its drug cargo, methotrexate.
6. Discussion and future perspectives
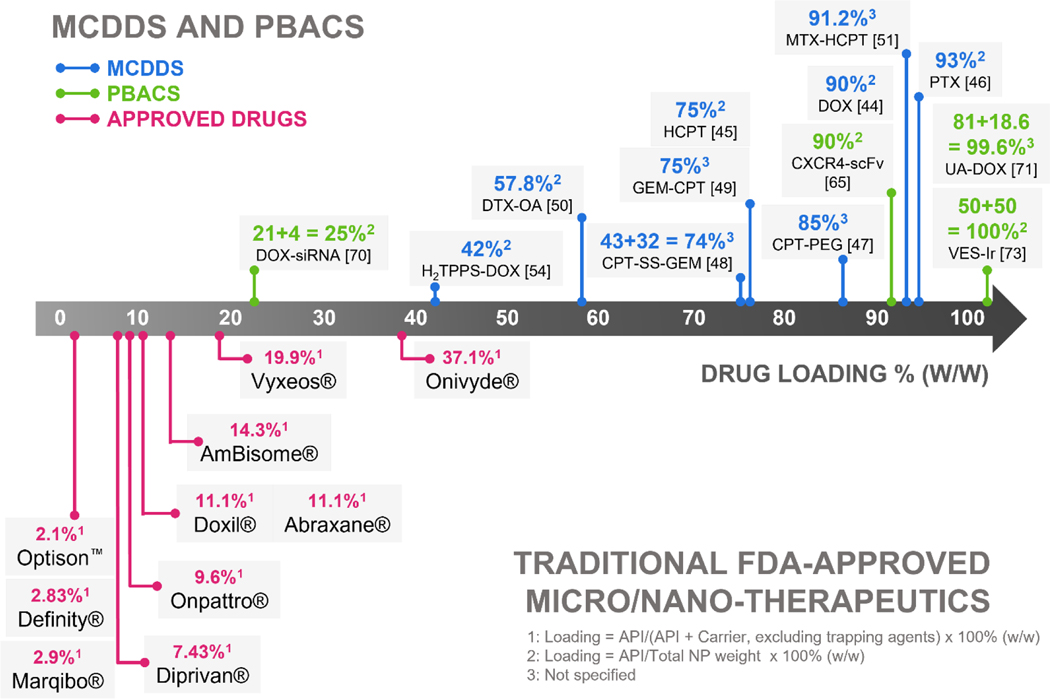
The emerging field of MCDDS and PBACS is promising. In the majority of cases, MCDDS show substantially higher drug loading (40–90+%) than current clinically approved nano-therapeutics (Figure 4 and Table 3) and display enhanced therapeutic efficacy compared to free drugs. The majority of FDA-approved nano-therapeutics have a drug loading of <20% except for Onyvide® (liposomal irinotecan), which has a drug loading of 37.1 % w/w. This is mostly limited to the drug loading/encapsulation strategies available to standard nano-formulations [76]. Alternatives to improve drug loading often involved the synthesis of lipid-drug conjugates [77]. Still, a combined high drug loading of over 20% is hard to reach.
Figure 4.
Drug loading comparisons between MCDDS/PBACS and FDA-approved standard micro/nano-DDS. In general, MCDDS/PBACS display higher drug loading compared to FDA-approved standard micro/nano-DDS. Top row: Representative MCDDS and PBACS organized by increasing drug loading. Bottom row: traditional FDA-approved nano-DDS with drug loading expressed as % w/w.
Table 3.
Drug loading of traditional FDA-approved micro/nano-DDS.
| Brand Name | Size | API | Carrier Excipient | Drug Loading (% w/w)† |
|---|---|---|---|---|
| Optison™ Microsphere |
3–4.5 μm | C3F8 (0.22 mg) | Human Albumin (10 mg) | 2.1% |
| Definity® Lipid Microsphere |
1–3.3 μm | C3F8 (6.52 mg/mL) | DPPA (0.045 mg/mL) DPPC (0.401 mg/mL) DPPE-mPEG5k (0.304 mg/mL) Propylene glycol (103.5 mg/mL) Glycerin (126.2 mg/mL) |
2.83% |
| Marqibo® Liposome |
100–140 nm |
Vincristine sulfate (5 mg) | Sphingomyelin (73.5 mg) Chol (29.5 mg) Trapping Agent: Citric Acid (33.6 mg/mL) Sodium Citrate (35.4 mg/mL) |
2.9% |
| Diprivan® Emulsion |
<200 nm | Propofol (10 mg/mL) | Soybean Oil (100 mg/mL) Glycerol (22.5 mg/mL) Egg Lecithin (12 mg/mL) |
7.43% |
| Onpattro® Lipid Complex | N/A | Patisiran siRNA (2 mg/mL) | Chol (6.2 mg/mL) DLin-MC3-DMA (13 mg/mL) DMG-mPEG2K (1.6 mg/mL) |
9.6% |
| Abraxane® | 130 nm | Paclitaxel (5 mg/mL) | Human Albumin (45 mg/mL) | 11.1% |
| Doxil® Liposome |
100 nm | Doxorubicin HCl (2 mg/mL) | HSPC (9.58 mg/mL) Chol (3.19 mg/mL) DSPE-mPEG (3.19 mg/mL) Trapping Agent: (NH4)2SO4 (2 mg/mL) |
11.1% |
| AmBisome® Liposome |
100 nm | Amphotericin B (50 mg) | HSPC (213 mg) DSPG (84 mg) Chol (52 mg) α-Tocopherol (0.64 mg) |
14.3% |
| Vyxeos® Liposome |
100 nm | Daunorubicin (44 mg) Cytarabine (100 mg) 1: 5 (m/m) |
DSPC (454 mg) DSPG (132 mg) Chol (32 mg) Trapping Agent: Copper Gluconate (100 mg) Triethanolamine (4 mg) |
19.9% |
| Onivyde® Liposome |
110 nm | Irinotecan free base (4.3 mg/mL) | DSPC (6.8 mg/mL) Chol (2.22 mg/mL) DSPE-mPEG2k (0.12 mg/mL) Trapping Agent: Sucrosofate potassium (2.02 mg/mL) |
37.1% |
Drug loading % (w/w) = API/(API + Carrier, excluding Trapping Agent) x 100 %
Through MCDDS and PBACS approaches, most achieved a drug loading of >40%, with many having a drug loading >70% (Figure 4 upper panel). The exception is the DOX-siRNA nanoparticle delivery system which needed polymer PEG coating used for improved circulation times, yet a combined 25% drug (DOX + siRNA) loading still outmatches most clinically approved standard nanotherapeutics, such as Doxil (11.1% DOX) and Onpattro (9.6% siRNA) [70].
MCDDS can be formed through simple methods such as self-assembly with amphiphilic drugs or self-assembly using functional linker groups. These systems are easily modified and can be engineered for many different purposes. Although these systems can improve the delivery of small molecules, nucleic acids, and proteins, several issues remain that must be overcome for successful clinical translation. The main issue with MCDDS is their limited stability due to the lack of a protective shell or carrier matrix. Additionally, many of these systems only form in the presence of a hydrophilic and hydrophobic molecule. Thus, not all drug combinations can be formed into MCDDS or PBACS, depending on the formulation strategy.
Pharmacoactive carriers can improve pharmacokinetics and disease outcomes. Many involve adding targeting moieties or active coatings onto drug nanostructures. A few other approaches use nucleic acids as the pharmacoactive carrier material to modulate gene expression while at the same time delivering an additional therapeutic cargo such as a small molecule drug. To maximize the therapeutic efficacy of these systems, it is pertinent that the pharmacological activity of the carrier material is enhanced and that the formed system is sufficiently stable.
As a novel emerging field, MCDDS/PBACS nano-therapeutics are rapidly developing pre-clinically. Minimal-carrier (or even carrier-free) therapeutics are prevalent in clinical trials, although they are not always “nano”-formulated [25]. An example of this type of system is an antibody-drug conjugate that is “carrier-free” but does not form into a particle. There are also clinical trials using active carrier materials (such as proteins) that can both deliver and provide additional biological and pharmacological activities, in combination with polymers that are not pharmacoactive, often referred to as drug-polymer conjugates [78].
When long blood circulation is required and when stability is a concern for MCDDS, adding coatings such as PEG would provide steric shielding and prevent premature uptake by macrophages [44,46]. Linkers, such as ester bonds, can also be used to stabilize MCDDS, as these induce drug assembly into nanoparticles without the need for large amounts of carrier material [59]. However, each time new materials are added, the loading efficiency of the MCDDS will decrease. In addition, the geometry of MCDDS and PBACS, such as rods, triangles, and cubes, is an area that could be further explored to improve their stability. For example, rod geometries have been shown to have superior stability to spherical DDS, but this geometry is only useful for specific applications where rods are desired [10]. Complex geometry may improve stability but could also make manufacturing more difficult, which could be an issue for clinical translation.
An increasingly important aspect in nano-drug delivery is the balance between drug loading versus the number of nanoparticles, and which is more critical for the successful delivery of nanoparticles to the disease site. Specifically, Ouyang et al. recently demonstrated that the number of nanoparticles delivered is an important factor in drug delivery success [80]. This is because Kupffer cells in the liver must be saturated before the nanoparticles can reach the tumor. This study aimed to determine a nanoparticle number threshold for successful delivery into tumors. Using both experimental methods and meta-analysis of previously approved versus unapproved nanoparticle systems, they determined a threshold of 1 billion particles for successful tumor delivery for both unloaded nanoparticles and nanoparticles loaded with therapeutics. When designing nanoparticle systems, should we focus on increasing drug loading, as discussed in this review, or on increasing the nanoparticle number? It will be important to consider balancing the risk of carrier materials and materials toxicity with the number of nanoparticles needed to overwhelm macrophages. If carrier materials do not show significant toxicity as a side effect, the benefits of dosing more nanoparticles for delivery may exceed the risks. The long-term toxicity of carrier materials needs to be studied more in-depth to understand the risk-benefit balance. Furthermore, high drug loading may be more efficient if there is a way to saturate macrophages with inert, non-toxic filler nanoparticles followed by a dose of high drug-content nanoparticles. If the findings of Ouyang et al. hold true, saturating macrophages with inert, non-toxic filler nanoparticles would also help to boost tumor accumulation of MCDDS. Additionally, it will be important to understand if saturating liver macrophages will also help to deliver nanoparticles and MCDDS to other non-tumor organs and tissues, such as the heart. Systemic delivery of nanoparticles and therapeutics specifically to the site of disease has been a constant struggle, and unfortunately, progress has been slow.
Similar to traditional nanoparticles, the majority of MCDDS have been developed to target tumors, and there is an opportunity gap for other diseases. For example, systemic nanoparticle delivery to the heart remains highly inefficient, and accumulation is low. One paper found that nanoparticles in the range of 20–200 nm could reach the damaged heart tissue, and by increasing nanoparticle size, accumulation increased [81]. Additionally, more nanoparticles accumulated in the heart following ischemia-reperfusion injury compared to a healthy heart, likely due to the EPR effect in diseased cardiac tissue [82]. Commonly used strategies such as PEGylation and targeting approaches have been used to increase targeting of traditional nano-carriers to the heart and could be applied to MCDDS and PBACS [83].
A less explored area is the use of MCDDS and PBACS for imaging, which tends to benefit from a high loading of the imaging agent, such as a dye, per particle to ensure sufficient brightness. For instance, Zhang et al. prepared a nanoparticle by mixing a surfactant with a chromophore and achieved a 3:1 chromophore to surfactant ratio [84]. This system was designed for imaging the intestinal tract and consisted of hydrophobic napthalocyanine dyes. These self-assembled into micelles and could be used as a photoacoustic imaging technique that did not degrade in the intestinal environment when administered orally. Another study by Shamay et al. formulated self-assembled particles of drugs and dyes to image various cancers [85]. They used sulfated indocyanine dye as the active carrier, which could self-assemble into nanoparticles and encapsulate hydrophobic drugs. They used sorafenib and trametinib as hydrophobic anti-tumor drugs, and these both targeted and inhibited tumor growth. They showed drug loading of up to 90% and did not use a traditional protective carrier.
Currently, renal-clearable nano-DDS, like silica and noble metal NPs, are commonly used for medical imaging [86]. These particles are ultra small (<8 nm) and reduce systemic exposure due to their quick tumor penetration. They could be considered as another alternative to traditional DDS since they have a fast degradation and clearance from the body, which reduces the chance of an immune response compared to other carrier materials. However, due to their small size, renal-clearable nano-DDS generally have lower drug loading compared to MCDDS [86]. Lastly, MCDDS has the potential to be renal clearable if they are designed to be <10 nm and <50 kDa [87][88].
MCDDS are often formulated through self-assembly, a process that can be unpredictable and that relies heavily on the drugs’ properties such as solubility, hydrophobicity, and other factors. Computational modeling and machine learning can be used to help predict the structures of MCDDS and PBACS and aid in formulating systems with optimized loading and defined shapes and size. For example, Shamay et al. used machine learning algorithms to first predict which molecular descriptors were correlated with stable self-assembled structures using retrospective quantitative structure-property relationship analysis [85]. They also performed molecular dynamics simulations with the top-performing structures to predict assembly at the atomic level. As the technology matures, it could become invaluable in the creation of MCDDS and PBACS with both high stability and drug loading.
MCDDS and PBACS can also be incorporated into other systems for a wide range of applications. They can be conjugated into patches or hydrogels to improve the stability and specificity of drug targeting. MCDDS and PBACS offer unique ways to engineer multi-functional drug delivery systems whilst minimizing the amount of material to be metabolized. Since MCDDS do not require the manufacturing of a carrier to deliver drugs, less material is needed to form the construct, which could help to decrease manufacturing costs. Furthermore, if properly formulated, fewer MCDDS and PBACS are required due to enhanced loading and increased potency, which could further decrease costs associated with materials and production.
In summary, MCDDS and PBACS are promising approaches to minimize waste in drug delivery systems and to avoid the unnecessary sequelae of inert carrier materials. These systems ensure that the majority of the components in a formulation are pharmacologically active to increase therapeutic efficacy and reduce potential side effects. While the stability of these systems must be improved for clinical success, these drug delivery systems have great potential to improve drug efficacy and patient outcomes.
Highlights.
Nanosized, minimal-carrier and pharmacoactive delivery systems are an emerging trend in response to poor drug loading and toxicity concerns associated with traditional nanoparticles.
Drug loading, stability, and modifications to synthesize triggerable minimal-carrier nanoparticle systems are discussed.
Various pharmacoactive and bioactive carrier materials are presented, including peptide-based, nucleic acid based, and small molecule scaffold-based materials.
Acknowledgment
This review is dedicated to Frank Szoka in honor of his lifetime achievements and significant contributions to the field of drug delivery. This work is supported through funding by the NIH (R01EB023262, R01CA241679 and R21GM135853). Parts of figures 1, 2, and 3 were created with BioRender.
Declaration of Competing Interest
JN is an inventor on the patent applications for the EXO-Code technology that have been licensed to Exopharm and has received royalties. These relationships have been disclosed to and are under management by UNC-Chapel Hill.
Table of Abbreviations
- AIEgen
Aggregation-induced emission luminogen
- API
Active Pharmaceutical Ingredient
- Cb
Chlorambucil
- DDS
Drug Delivery System
- Ce6
Chlorin e6
- CPT
Camptothecin
- CSAN
Chemically self-assembled antibody nanoring
- CXCR4
C-X-C chemokine receptor type 4
- DCA
1,2-dicarboxylic-cyclohexene anhydride
- DOX
Doxorubicin
- DTX
Docetaxel
- DSPE
1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine
- DSPG
Distearoyl phosphatidylglycerol
- EPR
Enhanced Permeability and Retention
- GEM
Gemcitabine
- HA
Hyaluronic acid
- HER2
Human epidermal growth factor receptor 2
- HCPT
Hydroxycamptothecin
- HSPC
Hydrogenated Soy Phosphatidylcholine
- ICD
Immunogenic cell death
- Ir
Irinotecan
- MAN
Mannose
- MCDDS
Minimal-Carrier Drug Delivery System
- MDR
Multi-drug resistance
- MTX
Methotrexate
- NIR
Near-infrared
- NP
Nanoparticle
- NR
Nanorod
- OA
Oleic acid
- PBACS
Pharmacoactive and Bioactive Carrier System
- PBS
Phosphate Buffered Saline
- PDA
Polydopamine
- PD-L1
Programmed death-ligand 1
- PDT
Photodynamic therapy
- PEG
Polyethylene Glycol
- PNA
Peptide nucleic acid
- PTX
Paclitaxel
- RBM
RNA binding motif
- RNAi
RNA interference
- scFv
Single chain variable fragment
- siRNA
Small interfering RNA
- SS
Disulfide bond
- UA
Ursolic acid
- VES
Vitamin E succinate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bor G, Diana I, Azmi M, Yaghmur A, Nanomedicines for cancer therapy : current status, challenges and future prospects, 10 (2019) 113–132. [DOI] [PubMed] [Google Scholar]
- [2].Marchal S, Hor E, Millard M, Bezdetnaya L, Anticancer Drug Delivery : An Update on Clinically Applied Nanotherapeutics, (2015) 1601–1611. 10.1007/s40265-015-0453-3. [DOI] [PubMed] [Google Scholar]
- [3].Loar RW, V Noel C, Tunuguntla H, Colquitt JL, Pignatelli RH, State of the art review : Chemotherapy-induced cardiotoxicity in children, (2018) 5–15. 10.1111/chd.12564. [DOI] [PubMed] [Google Scholar]
- [4].Nguyen J, Szoka FC, Nucleic acid delivery: The missing pieces of the puzzle?, Acc. Chem. Res 45 (2012) 1153–1162. 10.1021/ar3000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nguyen J, Steele TWJ, Merkel O, Reul R, Kissel T, Fast degrading polyesters as siRNA nano-carriers for pulmonary gene therapy, J. Control. Release 132 (2008) 243–251. 10.1016/j.jconrel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nguyen J, Walsh CL, Motion JPM, Perttu EK, Szoka F, Controlled nucleation of lipid nanoparticles., Pharm. Res 29 (2012) 2236–2248. 10.1007/s11095-012-0752-2. [DOI] [PubMed] [Google Scholar]
- [7].Nanomedicine and the COVID-19 vaccines, Nat. Nanotechnol 15 (2020) 963. 10.1038/s41565-020-00820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moghimi SM, Hunter AC, Andresen TL, Factors Controlling Nanoparticle Pharmacokinetics : An Integrated Analysis and Perspective, (2012) 481–506. 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- [9].Silverman JA, Deitcher SR, Marqibo Ò ( vincristine sulfate liposome injection ) improves the pharmacokinetics and pharmacodynamics of vincristine, (2013) 555–564. 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Han H, The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction, 11 (2016) 673–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Colby AH, Oberlies NH, Pearce CJ, Herrera VLM, Colson YL, Grinstaff MW, systems for peritoneal cancers : a case study of the design, characterization and development of the expansile nanoparticle, 9 (2017) 1–20. 10.1002/wnan.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Blanco E, Shen H, Ferrari M, perspective Principles of nanoparticle design for overcoming biological barriers to drug delivery, 33 (2015) 941–952. 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Meyer RA, Green JJ, Shaping the future of nanomedicine : anisotropy in polymeric nanoparticle design, 8 (2016) 191–207. 10.1002/wnan.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rytting E, Nguyen J, Wang X, Kissel T, Biodegradable polymeric nanocarriers for pulmonary drug delivery, Expert Opin. Drug Deliv 5 (2008) 629–639. 10.1517/17425247.5.6.629. [DOI] [PubMed] [Google Scholar]
- [15].Omlor JA, Nguyen J, Bals R, Dinh QT, Nanotechnology in respiratory medicine, Respir. Res 16 (2015) 1–9. 10.1186/s12931-015-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chavanpatil MD, Khdair A, Patil Y, Handa H, Mao G, PHARMACEUTICAL NANOTECHNOLOGY Polymer-Surfactant Nanoparticles for Sustained Release of Water-Soluble Drugs, J. Pharm. Sci 96 (2007) 3379–3389. 10.1002/jps.20961. [DOI] [PubMed] [Google Scholar]
- [17].Li F, Shi X, Cubic phase nanoparticles for sustained release of ibuprofen : formulation, characterization, and enhanced bioavailability study, (2013) 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang J, Seo MJ, Deci MB, Weil BR, Canty JM, Nguyen J, Effect of CCR2 inhibitor-loaded lipid micelles on inflammatory cell migration and cardiac function after myocardial infarction, Int. J. Nanomedicine 13 (2018) 6441–6451. 10.2147/IJN.S178650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ravi Kumar MNV, Bakowsky U, Lehr CM, Preparation and characterization of cationic PLGA nanospheres as DNA carriers, Biomaterials. 25 (2004) 1771–1777. 10.1016/j.biomaterials.2003.08.069. [DOI] [PubMed] [Google Scholar]
- [20].Mishra D, Hubenak JR, Mathur AB, Review Article Nanoparticle systems as tools to improve drug delivery and therapeutic efficacy, (2013) 3646–3660. 10.1002/jbm.a.34642. [DOI] [PubMed] [Google Scholar]
- [21].Barenholz Y, Doxil® - The first FDA-approved nano-drug: Lessons learned, J. Control. Release 160 (2012) 117–134. 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- [22].Meza M, Greener Y, Hunt R, Perry B, Revall S, Barbee W, Murgo JP, Cheirif J, Myocardial contrast echocardiography: Reliable, safe, and efficacious myocardial perfusion assessment after intravenous injections of a new echocardiographic contrast agent, Am. Heart J 132 (1996) 871–881. 10.1016/S0002-8703(96)90324-5. [DOI] [PubMed] [Google Scholar]
- [23].Moreno-Aspitia A, Perez EA, Nanoparticle albumin-bound paclitaxel (ABI-007): A newer taxane alternative in breast cancer, Futur. Oncol 1 (2005) 755–762. 10.2217/14796694.1.6.755. [DOI] [PubMed] [Google Scholar]
- [24].Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, Stuart RK, Strickland SA, Hogge D, Solomon SR, Stone RM, Bixby DL, Kolitz JE, Schiller GJ, Wieduwilt MJ, Ryan DH, Hoering A, Banerjee K, Chiarella M, Louie AC, Medeiros BC, Cpx-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia, J. Clin. Oncol 36 (2018) 2684–2692. 10.1200/JCO.2017.77.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Anselmo AC, Mitragotri S, Nanoparticles in the clinic : An update, Bioeng. Transl. Med (2019) 1–16. 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chu KS, Schorzman AN, Finniss MC, Bowerman CJ, Peng L, Luft JC, Madden AJ, Wang AZ, Zamboni WC, Desimone JM, Biomaterials Nanoparticle drug loading as a design parameter to improve docetaxel pharmacokinetics and ef fi cacy, Biomaterials. 34 (2013) 8424–8429. 10.1016/j.biomaterials.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cai K, He X, Song Z, Yin Q, Zhang Y, Uckun FM, Jiang C, Cheng J, Dimeric Drug Polymeric Nanoparticles with Exceptionally High Drug Loading and Quantitative Loading Efficiency, (2015). 10.1021/ja513034e. [DOI] [PubMed] [Google Scholar]
- [28].Shen S, Wu Y, Liu Y, Wu D, High drug-loading nanomedicines: progress, current status, and prospects., Int. J. Nanomedicine 12 (2017) 4085–4109. 10.2147/IJN.S132780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang N, Cheng X, Li N, Wang H, Chen H, Nanocarriers and Their Loading Strategies, Adv. Healthc. Mater 8 (2019) 1801002. 10.1002/adhm.201801002. [DOI] [PubMed] [Google Scholar]
- [30].Liu Y, Yang G, Jin S, Xu L, Zhao C-X, Development of High-Drug-Loading Nanoparticles, Chempluschem. 85 (2020) 2143–2157. 10.1002/cplu.202000496. [DOI] [PubMed] [Google Scholar]
- [31].Charrois GJR, Allen TM, Rate of biodistribution of STEALTH R liposomes to tumor and skin : influence of liposome diameter and implications for toxicity and therapeutic activity, 1609 (2003) 102–108. [DOI] [PubMed] [Google Scholar]
- [32].Qin SY, Zhang AQ, Cheng SX, Rong L, Zhang XZ, Drug self-delivery systems for cancer therapy, Biomaterials. 112 (2017) 234–247. 10.1016/j.biomaterials.2016.10.016. [DOI] [PubMed] [Google Scholar]
- [33].Yang MY, Zhao RR, Fang YF, Jiang JL, Yuan XT, Shao JW, Carrier-free nanodrug: A novel strategy of cancer diagnosis and synergistic therapy, Int. J. Pharm 570 (2019) 118663. 10.1016/j.ijpharm.2019.118663. [DOI] [PubMed] [Google Scholar]
- [34].Van Etten EWM, Bakker-woudenberg IAJM, controlled release Sterically stabilized amphotericin B-liposomes “ toxicity and biodistribution in mice, 37 (1995) 123–129. [Google Scholar]
- [35].Gupta R, Shea J, Scaife C, Shurlygina A, Rapoport N, Polymeric micelles and nanoemulsions as drug carriers : Therapeutic efficacy, toxicity, and drug resistance, J. Control. Release 212 (2015) 70–77. 10.1016/j.jconrel.2015.06.019. [DOI] [PubMed] [Google Scholar]
- [36].Lars D, Nichols JW, Miura S, Han Y, Mind the gap : A survey of how cancer drug carriers are susceptible to the gap between research and practice, J. Control. Release 172 (2013) 1045–1064. 10.1016/j.jconrel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mirshafiee V, Jiang W, Sun B, Wang X, Xia T, Facilitating Translational Nanomedicine via Predictive Safety Assessment, Mol. Ther 25 (2017) 1522–1530. 10.1016/j.ymthe.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Reul R, Nguyen J, Biela A, Marxer E, Bakowsky U, Klebe G, Kissel T, Biophysical and biological investigation of DNA nano-complexes with a non-toxic, biodegradable aminemodified hyperbranched polyester, Int. J. Pharm 436 (2012) 97–105. 10.1016/j.ijpharm.2012.06.065. [DOI] [PubMed] [Google Scholar]
- [39].He H, Liu L, Morin EE, Liu M, Schwendeman A, Survey of Clinical Translation of Cancer Nanomedicines - Lessons Learned from Successes and Failures Published as part of the Accounts of Chemical Research special issue “ Nanomedicine and Beyond; ”., (2019). 10.1021/acs.accounts.9b00228. [DOI] [PubMed] [Google Scholar]
- [40].Salvioni L, Rizzuto MA, Bertolini JA, Pandolfi L, Colombo M, Prosperi D, Thirty Years of Cancer Nanomedicine :, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang Q, Jiang Q, Li N, Dai L, Liu Q, Song L, Wang J, Li Y, Tian J, Ding B, Du Y, DNA Origami as an In Vivo Drug Delivery Vehicle for Cancer Therapy, ACS Nano. 8 (2014) 6633–6643. 10.1021/nn502058j. [DOI] [PubMed] [Google Scholar]
- [42].Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du X, Hope MJ, Madden TD, Mui BL, Semple SC, Tam YK, Ciufolini M, Witzigmann D, Kulkarni JA, van der Meel R, Cullis PR, The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs, Nat. Nanotechnol 14 (2019) 1084–1087. 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- [43].Cross R, Without these lipid shells, there would be no mRNA vaccines for COVID-19, Chem. Eng. News (2021) 1–9. https://cen.acs.org/pharmaceuticals/drug-delivery/Without-lipidshells-mRNA-vaccines/99/i8 (accessed September 9, 2021). [Google Scholar]
- [44].Yu C, Zhou M, Zhang X, Wei W, Chen X, Zhang X, Smart doxorubicin nanoparticles with high drug payload for enhanced chemotherapy against drug resistance and cancer diagnosis, Nanoscale. 7 (2015) 5683–5690. 10.1039/c5nr00290g. [DOI] [PubMed] [Google Scholar]
- [45].Yang X, Liu Y, Zhao Y, Han M, Guo Y, Kuang H, Wang X, A stabilizer-free and organic solvent-free method to prepare 10-hydroxycamptothecin nanocrystals: In vitro and in vivo evaluation, Int. J. Nanomedicine 11 (2016) 2979–2994. 10.2147/IJN.S102726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li Y, Yang Y, An F, Liu Z, Zhang X, Carrier-free, functionalized pure drug nanorods as a novel cancer-targeted drug delivery platform, (2012). 10.1088/0957-4484/24/1/015103. [DOI] [PubMed] [Google Scholar]
- [47].Zhou Z, Piao Y, Hao L, Wang G, Zhou Z, Shen Y, Acidity-responsive shell-sheddable camptothecin-based nanofibers for carrier-free cancer drug delivery, Nanoscale. 11 (2019) 15907–15916. 10.1039/c9nr03872h. [DOI] [PubMed] [Google Scholar]
- [48].Xu Y, Huang Y, Zhang X, Lu W, Yu J, Liu S, Carrier-free Janus nano-prodrug based on camptothecin and gemcitabine : Reduction-triggered drug release and synergistic in vitro antiproliferative effect in multiple cancer cells, Int. J. Pharm 550 (2018) 45–56. 10.1016/j.ijpharm.2018.08.041. [DOI] [PubMed] [Google Scholar]
- [49].Hou M, Xue P, Gao Y, Ma X, Bai S, Kang Y, Xu Z, Gemcitabine–camptothecin conjugates: a hybrid prodrug for controlled drug release and synergistic therapeutics, Biomater. Sci 5 (2017) 1889–1897. 10.1039/c7bm00382j. [DOI] [PubMed] [Google Scholar]
- [50].Jing F, Guo Q, Xu W, Qu H, Sui Z, Docetaxel prodrug self-assembled nanosystem : Synthesis, formulation and cytotoxicity, Bioorg. Med. Chem. Lett 28 (2018) 826–830. 10.1016/j.bmcl.2017.07.041. [DOI] [PubMed] [Google Scholar]
- [51].Li Y, Lin J, Cai Z, Wang P, Luo Q, Yao C, Tumor microenvironment-activated self-recognizing nanodrug through directly tailored assembly of small-molecules for targeted synergistic chemotherapy, J. Control. Release 321 (2020) 222–235. 10.1016/j.jconrel.2020.02.025. [DOI] [PubMed] [Google Scholar]
- [52].Hou M, Gao Y, Shi X, Bai S, Ma X, Li B, Xiao B, Methotrexate-based amphiphilic prodrug nanoaggregates for co-administration of multiple therapeutics and synergistic cancer therapy, Acta Biomater. 77 (2018) 228–239. 10.1016/j.actbio.2018.07.014. [DOI] [PubMed] [Google Scholar]
- [53].Chen F, Zhao Y, Pan Y, Xue X, Zhang X, Kumar A, Liang X, Synergistically Enhanced Therapeutic Effect of a Carrier-Free HCPT/DOX Nanodrug on Breast Cancer Cells through Improved Cellular Drug Accumulation, Mol. Pharm 12 (2015) 2237–2244. 10.1021/mp500744m. [DOI] [PubMed] [Google Scholar]
- [54].Liu L, Bao Y, Wang J, Xiao C, Chen L, Construction of carrier-free porphyrin-based drug self-framed delivery system to reverse multidrug resistance through, Dye. Pigment 177 (2020) 107922. 10.1016/j.dyepig.2019.107922. [DOI] [Google Scholar]
- [55].Zhao YY, Zhao YY, Ma Q, Zhang H, Liu Y, Hong J, Ding Z, Liu M, Han J, Novel carrier-free nanoparticles composed of 7-ethyl-10-hydroxycamptothecin and chlorin e6: Self-assembly mechanism investigation and in vitro/in vivo evaluation, Colloids Surfaces B Biointerfaces. 188 (2020) 110722. 10.1016/j.colsurfb.2019.110722. [DOI] [PubMed] [Google Scholar]
- [56].Zhang W, Wen Y, He D, Wang Y, Liu X, Li C, Liang X, Near-infrared AIEgens as transformers to enhance tumor treatment e ffi cacy with controllable self-assembled redox-responsive carrier-free nanodrug, Biomaterials. 193 (2019) 12–21. 10.1016/j.biomaterials.2018.12.007. [DOI] [PubMed] [Google Scholar]
- [57].Li M, Sun X, Zhang N, Wang W, Yang Y, Jia H, Liu W, NIR-Activated Polydopamine-Coated Carrier-Free “Nanobomb” for In Situ On-Demand Drug Release, Adv. Sci 5 (2018) 1–10. 10.1002/advs.201800155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Huang P, Wang D, Su Y, Huang W, Zhou Y, Cui D, Zhu X, Yan D, Combination of small molecule prodrug and nanodrug delivery: Amphiphilic drug-drug conjugate for cancer therapy, J. Am. Chem. Soc 136 (2014) 11748–11756. 10.1021/ja505212y. [DOI] [PubMed] [Google Scholar]
- [59].Pakdel M, Raissi H, Hosseini S, Evaluation the synergistic antitumor effect of methotrexate – camptothecin codelivery prodrug from self-assembly process to acid-catalyzed both drugs release : A comprehensive theoretical study, J. Comput. Chem 41 (2020) 1486–1496. 10.1002/jcc.26192. [DOI] [PubMed] [Google Scholar]
- [60].Zhu G, Mei L, Vishwasrao HD, Jacobson O, Wang Z, Liu Y, Yung BC, Fu X, Jin A, Niu G, Wang Q, Zhang F, Shroff H, Chen X, Intertwining DNA-RNA nanocapsules loaded with tumor neoantigens as synergistic nanovaccines for cancer immunotherapy, Nat. Commun 8 (2017). 10.1038/s41467-017-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhou Z, Piao Y, Hao L, Wang G, Zhou Z, Shen Y, Acidity-responsive shell-sheddable camptothecin-based nanofibers for carrier-free cancer drug delivery, Nanoscale. 11 (2019) 15907–15916. 10.1039/C9NR03872H. [DOI] [PubMed] [Google Scholar]
- [62].Merdan T, Kunath K, Petersen H, Bakowsky U, Voigt KH, Kopecek J, Kissel T, PEGylation of poly(ethylene imine) affects stability of complexes with plasmid DNA under in vivo conditions in a dose-dependent manner after intravenous injection into mice., Bioconjug. Chem 16 (2005) 785–792. 10.1021/bc049743q. [DOI] [PubMed] [Google Scholar]
- [63].Fegan A, Kumarapperuma SC, Wagner CR, Chemically self-assembled antibody nanostructures as potential drug carriers, Mol. Pharm 9 (2012) 3218–3227. 10.1021/mp300303k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Liu J, Song L, Liu S, Zhao S, Jiang Q, Ding B, A Tailored DNA Nanoplatform for Synergistic RNAi-/Chemotherapy of Multidrug-Resistant Tumors, Angew. Chemie - Int. Ed 57 (2018) 15486–15490. 10.1002/anie.201809452. [DOI] [PubMed] [Google Scholar]
- [65].Deci MB, Liu M, Gonya J, Lee CJ, Li T, Ferguson SW, Bonacquisti EE, Wang J, Nguyen J, Carrier-Free CXCR4-Targeted Nanoplexes Designed for Polarizing Macrophages to Suppress Tumor Growth, Cell. Mol. Bioeng 12 (2019) 375–388. 10.1007/s12195-019-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Xin X, Teng C, Du X, Lv Y, Xiao Q, Wu Y, He W, Yin L, Drug-delivering-drug platform-mediated potent protein therapeutics via a non-endo-lysosomal route, Theranostics. 8 (2018) 3474–3489. 10.7150/thno.23804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Liu Q, Wang D, Xu Z, Huang C, Zhang C, He B, Mao C, Wang G, Qian H, Targeted Delivery of Rab26 siRNA with Precisely Tailored DNA Prism for Lung Cancer Therapy, ChemBioChem. 20 (2019) 1139–1144. 10.1002/cbic.201800761. [DOI] [PubMed] [Google Scholar]
- [68].Liu Q, Wang D, Yuan M, He BF, Li J, Mao C, Wang GS, Qian H, Capturing intracellular oncogenic microRNAs with self-assembled DNA nanostructures for microRNA-based cancer therapy, Chem. Sci 9 (2018) 7562–7568. 10.1039/C8SC03039A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shu Y, Yin H, Rajabi M, Li H, Vieweger M, Guo S, Shu D, Guo P, RNA-based micelles: A novel platform for paclitaxel loading and delivery, J. Control. Release 276 (2018) 17–29. 10.1016/j.jconrel.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chen S, Li D, Du X, He X, Huang M, Wang Y, Yang X, Wang J, Carrier-free nanoassembly of doxorubicin prodrug and siRNA for combinationally inducing immunogenic cell death and reversing immunosuppression, Nano Today. 35 (2020) 100924. 10.1016/j.nantod.2020.100924. [DOI] [Google Scholar]
- [71].Jiang K, Han L, Guo Y, Zheng G, Fan L, Shen Z, Zhao R, Shao J, A carrier-free dual-drug nanodelivery system functionalized with aptamer specific targeting HER2-overexpressing cancer cells, J. Mater. Chem. B 5 (2017) 9121–9129. 10.1039/c7tb02562a. [DOI] [PubMed] [Google Scholar]
- [72].Taghdisi SM, Danesh NM, Ramezani M, Yazdian-Robati R, Abnous K, A Novel AS1411 Aptamer-Based Three-Way Junction Pocket DNA Nanostructure Loaded with Doxorubicin for Targeting Cancer Cells in Vitro and in Vivo, Mol. Pharm 15 (2018) 1972–1978. 10.1021/acs.molpharmaceut.8b00124. [DOI] [PubMed] [Google Scholar]
- [73].Ling L, Vitamin E-based prodrug self-delivery for nanoformulated irinotecan with synergistic antitumor therapeutics, Int. J. Pharm 577 (2020) 119049. 10.1016/j.ijpharm.2020.119049. [DOI] [PubMed] [Google Scholar]
- [74].Fan Z, Wang Y, Xiang S, Zuo W, Huang D, Jiang B, Sun H, Yin W, Xie L, Hou Z, Dual-self-recognizing, stimulus-responsive and carrier-free methotrexate–mannose conjugate nanoparticles with highly synergistic chemotherapeutic effects, J. Mater. Chem. B 8 (2020) 1922–1934. 10.1039/d0tb00049c. [DOI] [PubMed] [Google Scholar]
- [75].Swenson CS, Heemstra JM, Peptide nucleic acids harness dual information codes in a single molecule, Chem. Commun 56 (2020) 1926–1935. 10.1039/c9cc09905k. [DOI] [PubMed] [Google Scholar]
- [76].Gubernator J, Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity, Expert Opin. Drug Deliv 8 (2011) 565–580. 10.1517/17425247.2011.566552. [DOI] [PubMed] [Google Scholar]
- [77].Mei K-C, Liao Y-P, Jiang J, Chiang M, Khazaieli M, Liu X, Wang X, Liu Q, Chang CH, Zhang X, Li J, Ji Y, Melano B, Telesca D, Xia T, Meng H, Nel AE, Liposomal Delivery of Mitoxantrone and a Cholesteryl Indoximod Prodrug Provides Effective Chemoimmunotherapy in Multiple Solid Tumors, ACS Nano. 14 (2020) 13343–13366. 10.1021/acsnano.0c05194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Singh J, Desai S, Yadav S, Narasimhan B, Kaur H, Polymer Drug Conjugates: Recent Advancements in Various Diseases., Curr. Pharm. Des 22 (2016) 2821–2843. 10.2174/1381612822666160217125515. [DOI] [PubMed] [Google Scholar]
- [79].Zhao Y, Alakhova DY, Kim JO, Bronich TK, V Kabanov A, A simple way to enhance Doxil® therapy: drug release from liposomes at the tumor site by amphiphilic block copolymer, J. Control. Release 168 (2013) 61–69. 10.1016/j.jconrel.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ouyang B, Poon W, Zhang YN, Lin ZP, Kingston BR, Tavares AJ, Zhang Y, Chen J, Valic MS, Syed AM, MacMillan P, Couture-Senécal J, Zheng G, Chan WCW, The dose threshold for nanoparticle tumour delivery, Nat. Mater 19 (2020) 1362–1371. 10.1038/s41563-020-0755-z. [DOI] [PubMed] [Google Scholar]
- [81].Lundy DJ, Chen KH, Toh EKW, Hsieh PCH, Distribution of systemically administered nanoparticles reveals a size-dependent effect immediately following cardiac ischaemia-reperfusion injury, Sci. Rep 6 (2016) 1–10. 10.1038/srep25613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Prajnamitra RP, Chen HC, Lin CJ, Chen LL, Hsieh PCH, Nanotechnology approaches in tackling cardiovascular diseases, Molecules. 24 (2019). 10.3390/molecules24102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Nguyen J, Sievers R, Motion JPM, Kivimäe S, Fang Q, Lee RJ, Delivery of Lipid Micelles into Infarcted Myocardium Using a Lipid-Linked Matrix Metalloproteinase Targeting Peptide, Mol. Pharm 12 (2015) 1150–1157. 10.1021/mp500653y. [DOI] [PubMed] [Google Scholar]
- [84].Zhang Y, Jeon M, Rich LJ, Hong H, Geng J, Zhang Y, Shi S, Barnhart TE, Alexandridis P, Huizinga JD, Seshadri M, Cai W, Kim C, Lovell JF, Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines, Nat. Nanotechnol 9 (2014) 631–638. 10.1038/nnano.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shamay Y, Shah J, Işlk M, Mizrachi A, Leibold J, Tschaharganeh DF, Roxbury D, Budhathoki-Uprety J, Nawaly K, Sugarman JL, Baut E, Neiman MR, Dacek M, Ganesh KS, Johnson DC, Sridharan R, Chu KL, Rajasekhar VK, Lowe SW, Chodera JD, Heller DA, Quantitative self-assembly prediction yields targeted nanomedicines, Nat. Mater 17 (2018) 361–368. 10.1038/s41563-017-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Peng C, Huang Y, Zheng J, Renal clearable nanocarriers: Overcoming the physiological barriers for precise drug delivery and clearance, J. Control. Release 322 (2020) 64–80. 10.1016/j.jconrel.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ruggiero A, Villa CH, Bander E, Rey DA, Bergkvist M, Batt CA, Manova-Todorova K, Deen WM, Scheinberg DA, McDevitt MR, Paradoxical glomerular filtration of carbon nanotubes, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 12369–12374. 10.1073/pnas.0913667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Choi CHJ, Zuckerman JE, Webster P, Davis ME, Targeting kidney mesangium by nanoparticles of defined size, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 6656–6661. 10.1073/pnas.1103573108. [DOI] [PMC free article] [PubMed] [Google Scholar]