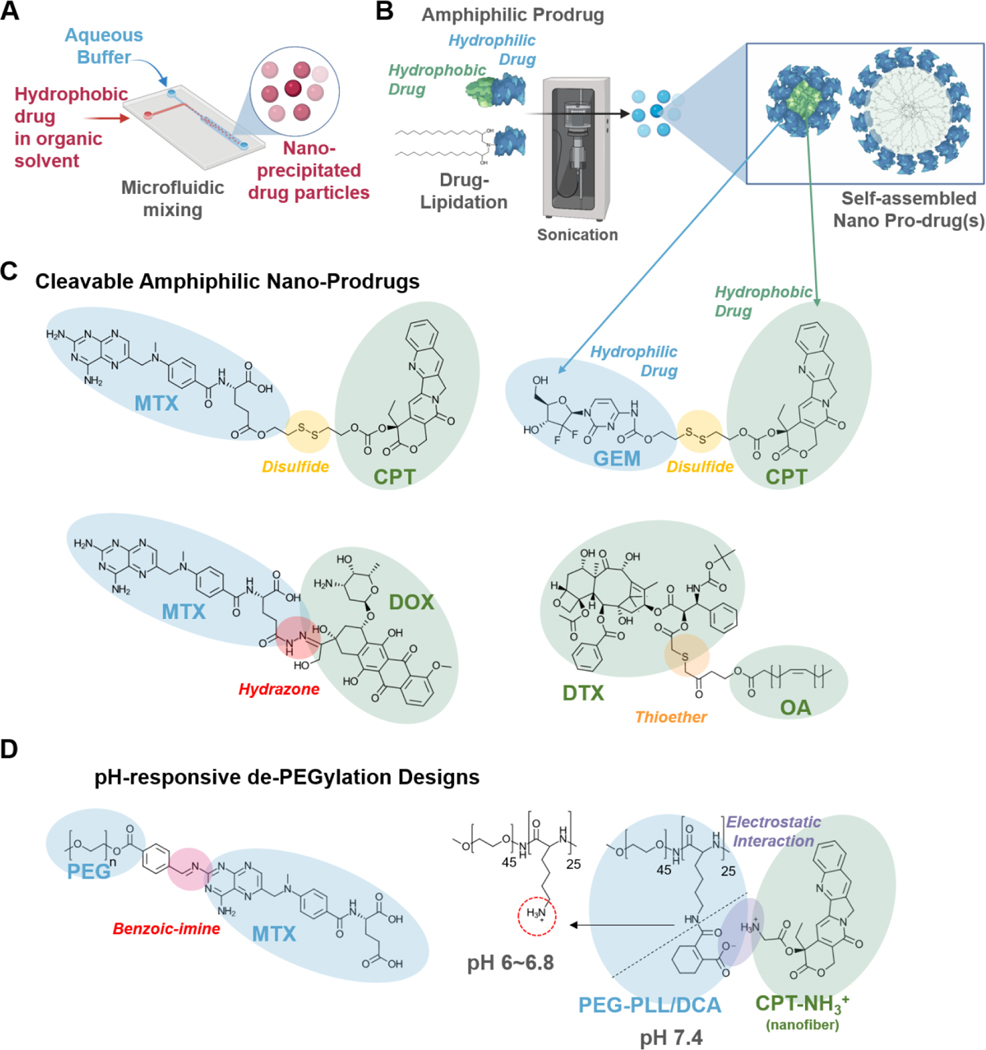
Figure 2. Formulation and synthetic approaches for high drug-loading MCDDS.
A) Microfluidic systems to rapidly and controllably precipitate hydrophobic drugs into drug nanoparticles in aqueous media. B) Amphiphilic self-assembly using hydrophilic-hydrophobic dual-drug conjugates or lipidation of hydrophilic drugs to form nano-prodrugs. C) Cleavable amphiphilic nano-prodrugs require a stimuli-responsive drug release mechanism to disassemble the nanocarrier and release the cargo. Such mechanisms include the use of redox and pH-sensitive disulfide, thioester, and hydrazone bonds. D) PEGylation is beneficial for nano-therapeutics, especially when extended blood circulation is required. However, PEGylation also hinders the cellular uptake of nanotherapeutics; thus, having pH-responsive de-PEGylation mechanisms could be advantageous to further improve the target specificity and efficacy of MCDDS. Such designs include acid liable benzoin-imine linkages [60], as well as cleavable DCA-capped PLL-b-PEG interactions [61].