Abstract
Objective
Systemic lupus erythematosus (SLE) patients are at higher risk of poor outcomes from coronavirus disease 2019 (COVID-19). The vaccination rate among such patients is unknown. We aimed to assess COVID-19 vaccine uptake among SLE patients.
Methods
We included 342 SLE patients from the Lupus Midwest Network and 350 age, sex, race, and county matched comparators. Vaccination uptake for influenza, pneumococcal, and zoster vaccines before pandemic restrictions began (up to February 29, 2020) was assessed. First-dose COVID-19 vaccine uptake was electronically retrieved and manually ascertained (December 15, 2020, to July 31, 2021). Time to COVID-19 vaccination, demographics, lupus manifestations, medications, comorbidity index, area deprivation index, and rurality measures were compared.
Results
On July 31, 2021, 83.3% of SLE patients and 85.5% of comparators were vaccinated against COVID-19. The COVID-19 vaccination rates were similar among SLE and comparators (hazard ratio: 0.93; 95% CI: 0.79–1.10). Non-vaccinated SLE patients were more likely to be men (27.3% versus 14.1% vaccinated), younger (mean 54.1 versus 58.8 years in vaccinated), have a shorter SLE duration (median 7.3 versus 10.7 years in vaccinated), and be less frequently vaccinated with influenza and pneumococcal vaccine.
Conclusion
SLE patients in the Lupus Midwest Network had similar COVID-19 vaccination uptake as matched comparators, most of whom were vaccinated early when the vaccine became available. One in six remain unvaccinated.
Keywords: Systemic lupus erythematosus, COVID-19, immunization, vaccine, vaccination, hesitancy
INTRODUCTION
Patients with autoimmune and inflammatory rheumatic diseases (AIIRD), including those with systemic lupus erythematosus (SLE), are at higher risk of poor outcomes from coronavirus disease 2019 (COVID-19).1 Vaccinations against COVID-19 have been shown to be safe and effective2-4 and first became available for use in the United States (US) on December 14, 2020. However, surveys of patients with SLE and other AIIRD have identified vaccine hesitancy in up to 50% of patients, which may be related to concerns about side effects, potential disease flares, or a lack of data among patients with SLE.5,6 As of February 13, 2022, the vaccination coverage with at least one dose in the US among those 12 years of age and older was above 85%.7 A case-control study among US veterans reported a mRNA COVID-19 vaccination coverage (≥1 dose) of 43% among immunocompromised patients during the first three months of vaccine availability,8 but the rate of vaccination and the factors associated with vaccination of patients with SLE are unknown. We aimed to assess the rate of COVID-19 vaccine uptake among patients with SLE as compared to a matched cohort of comparators.
METHODS
Study population
The US based Lupus Midwest Network (LUMEN  ) is a population-based registry of 27 counties in southeast Minnesota and southwest Wisconsin. It is part of the Rochester Epidemiology Project (REP),9 a record-linkage system which allows access to medical records from health care providers in the 27 counties.10,11 All patients with SLE who fulfilled the 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for SLE12 on January 1, 2015, in the 27-county area were included (see details of patient identification methods in supplemental material). Patients with SLE were matched by age, sex, race, and county of residence to non-SLE comparators from the REP in a 1:1 ratio. Both groups were followed until July 31, 2021, last medical encounter, or death. Those patients without medical encounters on or after December 15, 2020, were excluded. Any identified subjects who did not provide consent for use of their medical records for research purposes were excluded from the study. This study was approved by the institutional review boards of Mayo Clinic (20-006485) and Olmsted Medical Center (036-OMC-20).
) is a population-based registry of 27 counties in southeast Minnesota and southwest Wisconsin. It is part of the Rochester Epidemiology Project (REP),9 a record-linkage system which allows access to medical records from health care providers in the 27 counties.10,11 All patients with SLE who fulfilled the 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for SLE12 on January 1, 2015, in the 27-county area were included (see details of patient identification methods in supplemental material). Patients with SLE were matched by age, sex, race, and county of residence to non-SLE comparators from the REP in a 1:1 ratio. Both groups were followed until July 31, 2021, last medical encounter, or death. Those patients without medical encounters on or after December 15, 2020, were excluded. Any identified subjects who did not provide consent for use of their medical records for research purposes were excluded from the study. This study was approved by the institutional review boards of Mayo Clinic (20-006485) and Olmsted Medical Center (036-OMC-20).
Data collection
Through medical record review, we abstracted demographics, weight, height, and clinical SLE characteristics. Education level was electronically retrieved for patients with and without SLE. Immunosuppressant and immunomodulation therapy was retrieved for patients with SLE. Smoking status was electronically retrieved in August 2021. Charlson comorbidity index (CCI),13 excluding the rheumatological domain, was estimated using diagnostic codes for 5 years prior to December 15, 2020. Area deprivation index (ADI)14 at the census block group level were obtained using patient addresses. Rurality was assessed using the rural-urban commuting area (RUCA) codes.15 History of COVID-19 diagnosis or a positive test prior to December 15, 2020 was electronically retrieved. Vaccination uptake for seasonal influenza (at least once), pneumococcal, recombinant zoster (at least once) and COVID-19 vaccines was obtained from electronic medical records, which were complemented by information from the immunization information systems of Minnesota and Wisconsin.16 Vaccine administration data were abstracted for BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and Ad26.CoV2.S (Janssen), the three COVID-19 vaccines authorized in the US; receipt of the first dose was ascertained from December 15, 2020, to July 31, 2021.For the rest of vaccines, the uptake was evaluated from January 1, 2015, to February 29, 2020, just before the beginning of pandemic restrictions.
Statistical analysis
Descriptive statistics (means, percentages, etc.) were used to summarize the data. Chi-squared and Wilcoxon rank-sum tests were performed to compare the baseline characteristics between patients with SLE and comparators, and between vaccinated and unvaccinated patients with SLE. The cumulative incidence of vaccination was estimated for patients with SLE and comparators using Kaplan-Meier methods. Cox proportional hazards models with adjustment for age, sex, and race were used to compare vaccination rates between the two groups, and Poisson regression models were used to compare vaccination rates in the first four months to those after four months. Given the potential differences between groups following the exclusion of patients after matching, we performed a sensitivity analysis for COVID-19 vaccine uptake among those patients with SLE who kept their matched comparators. A p-value of <0.05 was considered statistically significant for all analyses. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The LUMEN registry included 465 patients with prevalent SLE and 465 non-SLE comparators matched on January 1, 2015. 120 SLE and 109 non-SLE did not have a medical encounter after December 15, 2020, when the COVID-19 vaccines became available and were not included in the analysis. In addition, 3 SLE and 6 non-SLE cases were excluded from the analysis since their vaccination data was not available. The study population included 342 patients with SLE and 350 non-SLE comparators (Table 1). SLE patients and comparators had a similar age, sex, racial/ethnic, and education level distribution. The ADI score was also similar in SLE and non-SLE comparators (mean 93.3 versus 93.6, respectively); and we did not observe differences in rurality with similar proportions of patients and comparators living in urban and rural areas (p=0.183) Patients with SLE had more comorbidities than comparators based on CCI scores (2.4 [SD 2.7] versus 1.6 [SD 2.3]; p<0.001). The mean body mass index (BMI) was similar between patients with SLE and comparators (29.6 [SD 7.8] versus 30.0 [SD 7.1] kg/m2, respectively), as well as the proportion of individuals with BMI above 30 and 40 kg/m2. Smoking status was also similar, 18.8% of SLE patients and 26.4% of comparators were current smokers (p=0.064). The history of COVID-19 diagnosis or having a positive test prior to the availability of COVID-19 vaccine was similar between patients with and without SLE (5% versus 6.3%, respectively; p=0.453). Previous vaccination for influenza and pneumococcus were significantly more frequent among patients with SLE than non-SLE comparators (Table 1).
TABLE 1.
Characteristics of patients with systemic lupus erythematosus (SLE) and non-SLE matched comparators, in the Lupus Midwest Network (LUMEN) registry on December 15, 2020.
| Characteristic | SLE N=342 |
Non-SLE N=350 |
p-value* |
|---|---|---|---|
| Age, years, mean (SD) | 57.9 (15.3) | 60.3 (15.3) | 0.051 |
| Sex, n (%) | 0.790 | ||
| Women | 285 (83.3) | 289 (82.6) | |
| Men | 57 (16.7) | 61 (17.4) | |
| Race/Ethnicity, n (%) | 0.943 | ||
| Non-Hispanic White | 297 (86.8) | 312 (89.1) | |
| Hispanic | 15 (4.4) | 14 (4.0) | |
| Non-Hispanic Black | 13 (3.8) | 9 (2.6) | |
| Non-Hispanic Asian | 12 (3.5) | 10 (2.9) | |
| Other/Mixed | 5 (1.5) | 5 (1.4) | |
| Education level, n (%) | 0.292 | ||
| Graduate school | 69 (21.3) | 71 (25.7) | |
| Technical school/College | 172 (53.1) | 137 (49.6) | |
| High school | 75 (23.1) | 56 (20.3) | |
| <High school | 8 (2.5) | 12 (4.3) | |
| Missing | 18 | 74 | |
| Area Deprivation Index, mean (SD) | 93.3 (12.5) | 93.6 (12.1) | 0.634 |
| Primary RUCA code, n (%) | 0.183 | ||
| Metropolitan area core | 135 (39.5) | 120 (34.3) | |
| Metropolitan area high commuting | 79 (23.1) | 80 (22.9) | |
| Micropolitan area core | 61 (17.8) | 53 (15.1) | |
| Micropolitan area high commuting | 15 (4.4) | 33 (9.4) | |
| Micropolitan area low commuting | 1 (0.3) | 2 (0.6) | |
| Small town core | 25 (7.3) | 26 (7.4) | |
| Small town high commuting | 4 (1.2) | 5 (1.4) | |
| Rural areas | 22 (6.4) | 31 (8.9) | |
| Charlson Comorbidity Index†, mean (SD) | 2.4 (2.7) | 1.6 (2.3) | <0.001 |
| BMI‡, kg/m2, mean (SD) | 29.6 (7.8) | 30.0 (7.1) | 0.166 |
| BMI >30 kg/m2, n (%)§ | 132 (40.1) | 128 (40.9) | 0.842 |
| BMI >40 kg/m2, n (%)§ | 35 (10.6) | 31 (9.9) | 0.759 |
| Missing, n (%) | 13 (3.8) | 37 (10.6) | |
| Smoking‡, n (%) | 0.064 | ||
| Current§ | 57 (18.8) | 74 (26.4) | |
| Former§ | 119 (39.1) | 92 (32.9) | |
| Never§ | 128 (42.1) | 114 (40.7) | |
| Missing | 38 (11.1) | 70 (20.0) | |
| History of COVID-19 diagnosis or positive test, n (%) | 17 (5.0) | 22 (6.3) | 0.453 |
| Previous vaccination ∥, n (%) | |||
| Influenza (at least once) | 271 (79.2) | 253 (72.3) | 0.033 |
| Pneumococcal vaccine | 167 (48.8) | 125 (35.7) | <0.001 |
| Recombinant zoster vaccine (at least once) | 60 (17.5) | 61 (17.4) | 0.968 |
Rank-sum or Chi-square test.
From December 15, 2015, to December 14, 2020, excluding rheumatologic category.
Upon entry to LUMEN cohort–January 1, 2015.
The denominator excludes missing.
Vaccine uptake from January 1, 2015 to February 29, 2020 for influenza and pneumococcal vaccine and from January 1, 2018 to February 29, 2020 for zoster vaccine
BMI: body mass index; RUCA: rural-urban commuting area; SD: standard deviation; SLE: systemic lupus erythematosus.
COVID-19 vaccine uptake
On July 31, 2021, the percentages of patients with SLE and non-SLE comparators with at least one dose of COVID-19 vaccine were 83.3% (95% CI: 78.6–86.9) and 85.5% (95% CI: 80.7–89.1), respectively, and this difference was not significant after adjustment for age, sex, and race (hazard ratio: 0.93; 95% CI: 0.79–1.1; p=0.4; Figure 1). By the fourth month of vaccine availability, more than 70% of the patients with SLE and the non-SLE comparators were vaccinated. In the sensitivity analysis, the vaccine uptake rates were similar among those SLE patients who kept their matched comparators, (83.6% [95% CI 78.3–87.7] and 85.3% [95% CI 79.6–89.5], respectively) (see supplemental table 1).
FIGURE 1.
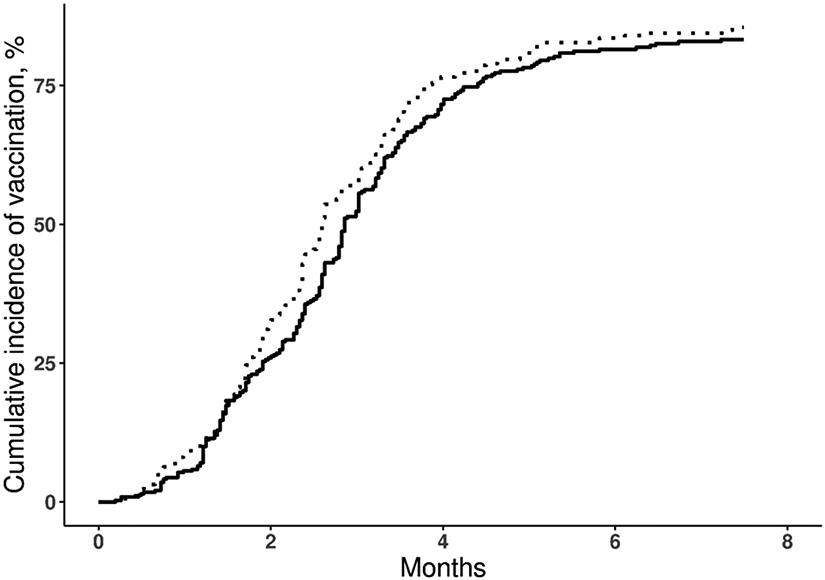
Kaplan-Meier curve showing the cumulative incidence of COVID-19 vaccination in patients with systemic lupus erythematosus (SLE, solid line) and non-SLE comparators (dashed line), from December 15, 2020, to July 31, 2021 (Hazard Ratio 0.93; 95% CI: 0.79–1.1).
Characteristics of unvaccinated SLE patients
Non-vaccinated patients with SLE were more likely to be men (27.3% versus 14.1% in vaccinated SLE patients, p=0.010), younger (mean 54.1 [SD 14.6] versus 58.8 [SD 15.4] years in vaccinated patients, p=0.022), and to have a shorter course of the disease (median duration 7.3 [IQR 2.6–13.4] versus 10.7 [IQR 4.7–20.3] years in vaccinated patients, p=0.008) (Table 2). There were no differences in racial distribution, education level, BMI, smoking status, CCI score, deprivation index, rurality measures, medications, or history of COVID-19 (Table 2). Non-vaccinated patients were also more likely to have a history of class II or V lupus nephritis (16.7% versus 8.0% in vaccinated patients, p=0.032), and were less frequently vaccinated with influenza and pneumococcal vaccine. No other SLE involvement was significantly different in relation to vaccine uptake.
TABLE 2.
Characteristics of patients with systemic lupus erythematosus according to COVID-19 vaccination status (≥1 dose) in the LUMEN Cohort on December 15, 2020.
| Characteristic | Vaccinated N=276 |
Not Vaccinated N=66 |
p-value* |
|---|---|---|---|
| Age, years, mean (SD) | 58.8 (15.4) | 54.1 (14.6) | 0.022 |
| Sex, n (%) | 0.010 | ||
| Women | 237 (85.9) | 48 (72.7) | |
| Men | 39 (14.1) | 18 (27.3) | |
| Race/Ethnicity, n (%) | 0.983 | ||
| Non-Hispanic White | 240 (87.0) | 57 (86.4) | |
| Hispanic | 12 (4.3) | 3 (4.5) | |
| Non-Hispanic Black | 11 (4.0) | 2 (3.0) | |
| Non-Hispanic Asian | 9 (3.3) | 3 (4.5) | |
| Other/mixed | 4 (1.5) | 1 (1.5) | |
| Education level, n (%) | 0.052 | ||
| Graduate school | 62 (23.7) | 7 (11.3) | |
| Technical school/College | 132 (50.4) | 40 (64.5) | |
| High school | 63 (24.0) | 12 (19.4) | |
| <High school | 5 (1.9) | 3 (4.8) | |
| Missing | 14 | 4 | |
| Area Deprivation Index, mean (SD) | 93.2 (12.8) | 94.0 (11.4) | 0.747 |
| Primary RUCA code, n (%) | 0.673 | ||
| Metropolitan area core | 115 (41.7) | 20 (30.3) | |
| Metropolitan area high commuting | 59 (21.4) | 20 (30.3) | |
| Micropolitan area core | 47 (17.0) | 14 (21.2) | |
| Micropolitan area high commuting | 12 (4.3) | 3 (4.5) | |
| Micropolitan area low commuting | 1 (0.4) | 0 (0.0) | |
| Small town core | 20 (7.2) | 5 (7.6) | |
| Small town high commuting | 3 (1.1) | 1 (1.5) | |
| Rural areas | 19 (6.9) | 3 (4.5) | |
| Charlson Comorbidity Index†, mean (SD) | 2.4 (2.7) | 2.5 (2.5) | 0.430 |
| BMI‡, kg/m2, mean (SD) | 29.4 (7.9) | 30.0 (7.3) | 0.396 |
| BMI >30 kg/m2, n (%)§ | 104 (39.5) | 28 (42.4) | 0.669 |
| BMI >40 kg/m2, n (%)§ | 27 (10.3) | 8 (12.1) | 0.662 |
| Missing, n (%) | 13 (4.7) | 0 (0.0) | |
| Smoking‡, n (%) | 0.726 | ||
| Current§ | 46 (19.0) | 11 (17.7) | |
| Former§ | 92 (38.0) | 27 (43.5) | |
| Never§ | 104 (43.0) | 24 (38.7) | |
| Missing | 34 (12.3) | 4 (6.1) | |
| History of COVID-19 diagnosis or positive test, n (%) | 12 (4.3) | 5 (7.6) | 0.278 |
| Previous vaccination∥, n (%) | |||
| Influenza (at least once) | 229 (83.0) | 42 (63.6) | <0.001 |
| Pneumococcal vaccine | 147 (53.3) | 20 (30.3) | <0.001 |
| Recombinant zoster vaccine (at least once) | 52 (18.8) | 8 (12.1) | 0.197 |
| SLE duration, years, median (IQR) | 10.7 (4.7–20.3) | 7.3 (2.6–13.4) | 0.008 |
| 2019 EULAR/ACR criteria ‡ | |||
| Constitutional, n (%) | |||
| Fever | 17 (6.2) | 5 (7.6) | 0.674 |
| Hematologic domain, n (%) | |||
| Leukopenia | 123 (44.6) | 26 (39.4) | 0.447 |
| Thrombocytopenia | 44 (15.9) | 10 (15.2) | 0.874 |
| Autoimmune hemolysis | 12 (4.3) | 2 (3.0) | 0.627 |
| Neuropsychiatric domain, n (%) | |||
| Delirium | 2 (0.7) | 0 (0.0) | 0.488 |
| Psychosis | 1 (0.4) | 0 (0.0) | 0.624 |
| Seizures | 5 (1.8) | 1 (1.5) | 0.869 |
| Mucocutaneous domain, n (%) | |||
| Non-scarring alopecia | 13 (4.7) | 2 (3.0) | 0.549 |
| Oral ulcers | 25 (9.1) | 5 (7.6) | 0.702 |
| Subacute cutaneous or discoid lupus | 45 (16.3) | 10 (15.1) | 0.819 |
| Acute cutaneous lupus | 68 (24.6) | 20 (30.3) | 0.344 |
| Serosal domain, n (%) | |||
| Pleural or pericardial effusion | 40 (14.5) | 13 (19.7) | 0.294 |
| Acute pericarditis | 26 (9.4) | 5 (7.6) | 0.639 |
| Musculoskeletal domain, n (%) | |||
| Arthritis | 186 (67.4) | 44 (66.7) | 0.910 |
| Renal domain, n (%) | |||
| Proteinuria >0.5g/24hrs | 64 (23.2) | 17 (25.8) | 0.659 |
| Class II or V lupus nephritis | 22 (8.0) | 11 (16.7) | 0.032 |
| Class III or IV lupus nephritis | 43 (15.6) | 13 (19.7) | 0.417 |
| Immunology domains, n (%) | |||
| Antiphospholipid antibodies | 65 (23.6) | 19 (28.8) | 0.375 |
| Low C3 or C4 | 105 (38.0) | 22 (33.3) | 0.477 |
| Low C3 and C4 | 89 (32.2) | 18 (27.3) | 0.434 |
| Anti-dsDNA | 203 (73.6) | 49 (74.2) | 0.909 |
| Anti-Smith | 57 (20.7) | 16 (24.2) | 0.523 |
| Immunosuppressant and immunomodulator drugs¶, n (%) | 239 (86.6) | 57 (86.4) | 0.961 |
| Hydroxychloroquine | 208 (75.4) | 47 (71.2) | 0.487 |
| Mycophenolate mofetil | 76 (27.5) | 17 (25.8) | 0.770 |
| Methotrexate | 47 (17.0) | 11 (16.7) | 0.944 |
| Azathioprine | 35 (12.7) | 8 (12.1) | 0.902 |
| Tacrolimus | 16 (5.8) | 4 (6.1) | 0.935 |
| Leflunomide | 10 (3.6) | 3 (4.5) | 0.725 |
| Chloroquine | 6 (2.2) | 3 (4.5) | 0.280 |
| Rituximab | 6 (2.2) | 1 (1.5) | 0.734 |
| Belimumab | 5 (1.8) | 2 (3.0) | 0.530 |
| Cyclophosphamide | 1 (0.4) | 1 (1.5) | 0.270 |
Rank-sum or Chi-square test.
From December 15, 2015, to December 14, 2020, excluding rheumatologic category.
Upon entry to LUMEN cohort–January 1, 2015.
The denominator excludes missing.
Vaccine uptake from January 1, 2015 to February 29, 2020 for influenza and pneumococcal vaccine and from January 1, 2018 to February 29, 2020 for zoster vaccine.
Medications which were used during a five-year lookback prior to December 15, 2020.
ACR: American College of Rheumatology; BMI: body mass index; EULAR: European League Against Rheumatism; IQR: interquartile range; RUCA: rural-urban commuting area; SD: standard deviation.
DISCUSSION
To our knowledge this is the first population-based study to document COVID-19 vaccine uptake among patients with SLE compared to a matched non-SLE population sample. In this study, 17% of patients with SLE were not vaccinated seven months after vaccines became available. Vaccine uptake with at least one dose was not higher among patients with SLE compared to the non-SLE comparators and similar to the current US vaccination coverage. Among SLE patients, COVID-19 vaccine uptake was lower in men, younger patients, and those with a shorter disease duration.
Patients with lupus are at a higher risk of severe COVID-19 as compared to the general population.1 Despite this elevated risk, in a prior international survey of 1266 patients with AIIRD performed before the COVID-19 vaccines were available, only 54% were willing to get vaccinated and 32% of the respondents were uncertain about getting a COVID-19 vaccine, but opinions may have evolved since then.5 In an Italian survey of patients with rheumatic diseases made during the first weeks of vaccine availability, 48% of patients with SLE who completed the survey declared willingness to receive COVID-19 vaccine.17 This may be related to concerns specific to this population, including rheumatic disease flares or a reduced effectiveness of vaccines due to concomitant immunosuppressive drugs.6,18 Additionally, high quality data for safety and efficacy of all three vaccines are available for the general population, but patients with rheumatic diseases were under-represented in these studies and may be waiting for data specific to people like them.2-4
While a vaccination rate of 83% as of July 31, 2021, may be viewed as encouraging7, the vaccination curve plateaued after a few months of vaccine availability. This trend was similar to comparators in this study and reflect broader observations of vaccine uptake in the US. In a prior report about vaccine hesitancy among patients with AIIRD, unvaccinated patients with SLE were more likely to be younger,6 but they did not find differences by sex or disease duration like we did. Both men and younger patients appear to be less likely to be vaccinated in the general population as well, suggesting that vaccination campaigns targeting these groups may be important, both for SLE and for the general population.19 We assessed social determinants of health with the ADI score and did not find any difference between vaccinated and unvaccinated patients with SLE. We did not find differences in the education level or the medications used by vaccinated and unvaccinated patients with SLE, nor with the previous diagnosis of COVID-19 infection, suggesting it was not a factor that affected the willingness to get vaccinated. But we did find a significant difference in the uptake for other vaccines, suggesting that vaccination hesitancy might be general and not exclusive to COVID-19 vaccine.
Strengths of this study include that the population-based data was retrieved up to July 31, 2021, a time when vaccines were widely available and most of the patients who wanted to get vaccinated would have had the opportunity to do so. In addition, access to medical records through the REP allowed us to confirm the SLE diagnosis and verify vaccination status regardless of where vaccinations were received. There are also limitations to our approach. LUMEN is based in the American upper midwest and may not be generalizable to other regions of the US. We did not observe differences among vaccinated and non-vaccinated patients with SLE based on race or ethnicity, but differences have been observed in the general population.19 Additional SLE-specific data (disease activity, damage, etc.) which might have an important role in the decision to COVID-19 vaccination, was not available at the time of the analysis. However, we did not find any difference in the CCI score (as a proxy of damage),20 between vaccinated and unvaccinated patients with SLE. Our study included patients with SLE who were diagnosed on or before January 1, 2015, and matching with comparators was performed on January 1, 2015, which may have led to some differences between groups at the time they received the vaccine. However, our sensitivity analysis demonstrated robustness of our results. We focused on coverage with at least one dose; we didn’t know the completeness of COVID-19 scheme or additional doses. Is possible (but unlikely) that some patients could have been vaccinated in other states. Conceivably, those SLE patients at earlier stages of their disease might have different vaccination rates. Finally, reasons for vaccination and non-vaccination were unknown.
In conclusion, most patients with SLE were vaccinated in the first four months after vaccinations became available. However, many patients with SLE remain unvaccinated despite a higher risk of severe COVID-19 outcomes. More studies are needed to evaluate how other factors like disease activity, or damage may influence the decision to get vaccinated or not against COVID-19 in patients with SLE. Our findings suggest that the COVID-19 vaccination uptake among patients with SLE is not different to the general population and suggest an opportunity to reduce vaccine hesitancy among those patients with SLE who are newly diagnosed, among men, and younger patients.
Supplementary Material
Funding Information:
The Lupus Midwest Network (LUMEN) project is supported by the Centers for Disease Control and Prevention of the U.S. Department of Health and Human Services (HHS) under Grant number U01 DP006491 as part of a financial assistance award totaling $1,750,000 with 100 percent funded by CDC/HHS. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS, or the U.S. Government.
The Rochester Epidemiology Project was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing Interests: None declared.
CDC Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
REFERENCES
- 1.D'Silva KM, Jorge A, Cohen A, et al. COVID-19 outcomes in patients with systemic autoimmune rheumatic diseases compared to the general population: a US multicenter, comparative cohort study. Arthritis Rheumatol 2021;73:914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med 2021;384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felten R, Dubois M, Ugarte-Gil MF, et al. Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol 2021;3:e243–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaur P, Agrawat H, Shukla A. COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: an interview-based survey. Rheumatol Int 2021;41:1601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. COVID-19 vaccinations in the United States. [Internet. Accessed February, 2022.] Available from: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total.
- 8.Young-Xu Y, Korves C, Roberts J, et al. Coverage and Estimated Effectiveness of mRNA COVID-19 Vaccines Among US Veterans. JAMA Netw Open 2021. ;4:e2128391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocca WA, Grossardt BR, Brue SM, et al. Data resource profile: Expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol 2018;47:368–j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 14.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N Engl J Med 2018;378:2456–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.USDA-ERS. Rural-urban commuting area codes. [Internet. Accessed October, 2021.] Available from: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/.
- 16.CDC. About immunization information systems. [Internet. Accessed October, 2021.] Available from: https://www.cdc.gov/vaccines/programs/iis/about.html.
- 17.Priori R, Pellegrino G, Colafrancesco S, et al. SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists. Ann Rheum Dis 2021;80:953–4. [DOI] [PubMed] [Google Scholar]
- 18.Figueroa-Parra G, Esquivel-Valerio JA, Santoyo-Fexas L, et al. Knowledge and attitudes about influenza vaccination in rheumatic diseases patients. Hum Vaccin Immunother 2021;17:1420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC. Demographic characteristics of people receiving COVID-19 vaccinations in the United States. [Internet. Accessed October, 2021.] Available from: https://covid.cdc.gov/covid-data-tracker/#vaccination-demographic.
- 20.Kim SK, Choe JY, Lee SS. Charlson comorbidity index is related to organ damage in systemic lupus erythematosus: data from KORean lupus Network (KORNET) registry. J Rheumatol 2017;44:452–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 )
)