Figure 7. Prevalence and treatment of NT5C2 Ser502 phosphorylation in relapse ALL patient-derived xenografts.
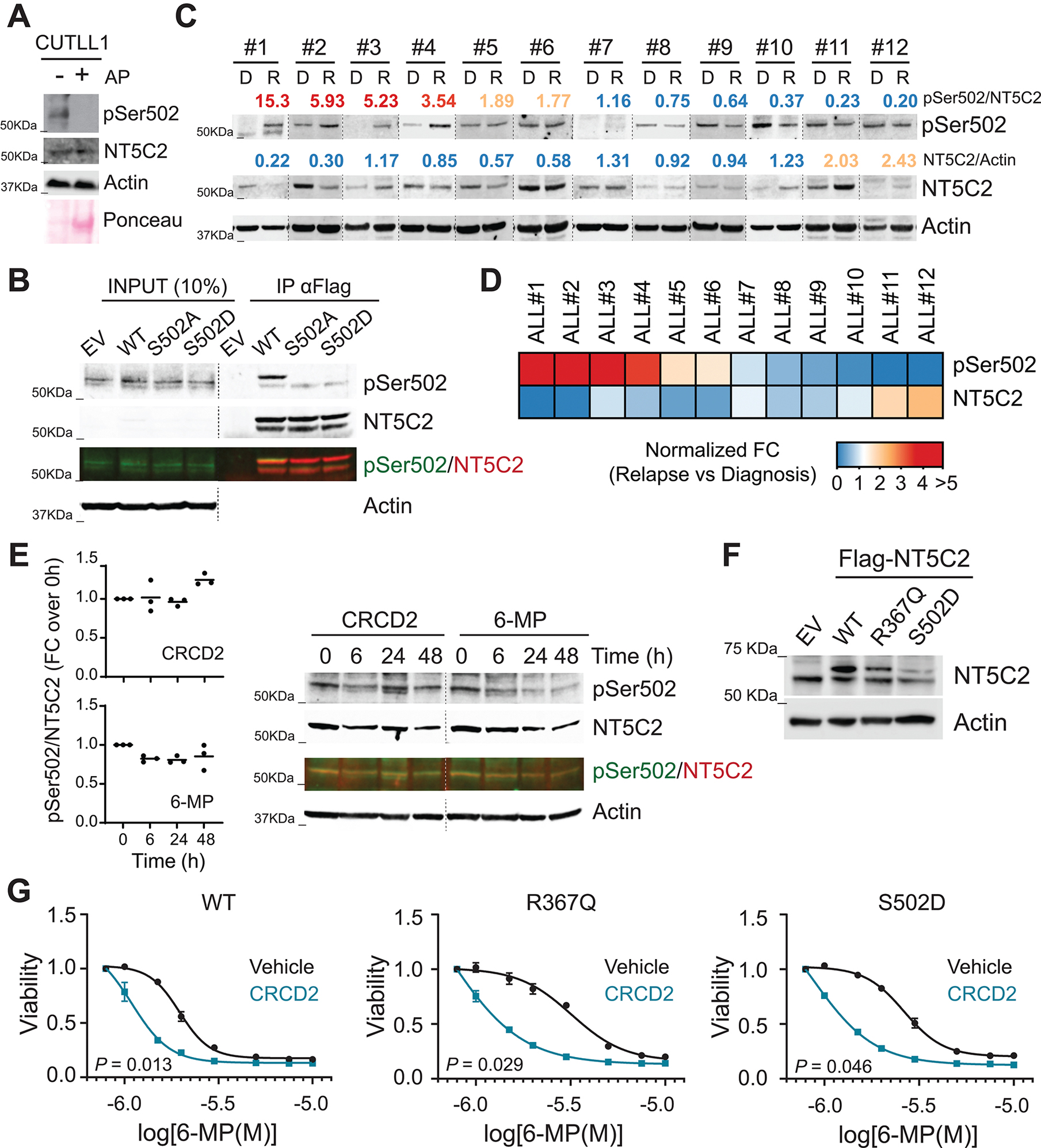
(A) Western-blot detection of NT5C2 pSer502 in CUTLL1 T-ALL cells. AP: alkaline phosphatase. (B) Western-blot detection of NT5C2 Ser502 phosphorylation after anti-Flag immunoprecipitation in Jurkat cells infected with empty vector or lentiviruses driving the expression of Flag-tagged wild-type, S502A and S502D mutants of NT5C2. Two additional experiments showed similar results. (C) NT5C2 Ser502 phosphorylation analysis in diagnostic and relapsed ALL patient derived xenografts. Numbers show normalized fold change of pSer502-NT5C2 and total levels of NT5C2 in relapse compared to the matched diagnosis xenograft. (D) Heatmap representation of normalized fold change of pSer502-NT5C2 and total levels of NT5C2 as in (C). (E) Western-blot detection of NT5C2 pSer502 levels in CUTLL1 cells treated with 10 μM CRCD2 or 1.5 μM 6-MP for 0, 6, 24 or 48 hours. A representative immuno-blot is shown. (F) Immunoblot analysis of Jurkat cells infected with empty vector or lentiviruses driving the expression of Flag-tagged wild-type, R367Q and S502D mutants of NT5C2. Expression levels were verified in three independent experiments. (G) Viability assay of Jurkat cells infected with wild-type or R367Q or S502D mutant NT5C2 expressing lentiviruses treated with vehicle or CRCD2 and increasing doses of 6-MP. Graphs show mean ± SD of three independent experiments performed in triplicate. P values were calculated using IC50 values and two-tailed Student’s t-test over wild-type.
