FIGURE 1.
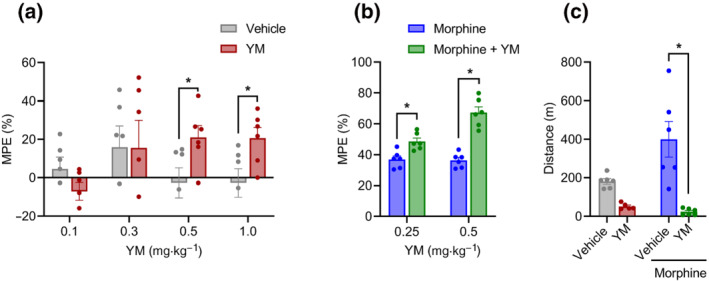
The effect of systemic subcutaneous administration of YM‐254890 (YM) on nociception and locomotion. (a) Dose–response effect of different concentrations of subcutaneous YM (0.1, 0.3, 0.5 and 1 mg·kg−1) and vehicle was tested on the hotplate test after 30 min of administration. Unpaired two‐tailed Student's t test of YM (0.5 mg·kg−1), P < 0.05, and YM (1.0 mg·kg−1), P < 0.05. (b) Effect of combined administration of subcutaneous YM (0.25 and 0.5 mg·kg−1) with a single dose of subcutaneous morphine (5 mg·kg−1) on the hotplate test after 30 min of morphine administration. YM was administered 10 min before the administration of morphine. Treatment: F (1, 10) = 59.06; dose: F (1, 10) = 13.21; and interaction: F (1, 10) = 12.08. Two‐way analysis of variance (ANOVA) with Bonferroni's post hoc test. (c) Changes in cumulative locomotor activity during 120 min of observation in open‐field test by subcutaneous administration of YM (0.5 mg·kg−1), morphine (5 mg·kg−1) and YM (0.5 mg·kg−1, 10 min before morphine) with morphine (5 mg·kg−1) and vehicle‐treated mice. Treatment: F (1, 19) = 26.02; effect of morphine: F (1, 19) = 3.71; and interaction: F (1, 19) = 6.2. Two‐way ANOVA with Bonferroni's post hoc test. In all panels, statistical analysis was performed combining both sexes, and significance was *P < 0.05; data sets (mean ± SEM) as analysed using two‐way ANOVA with Bonferroni's multiple comparisons test. MPE, maximum possible effect.
