Fig. 2. An unexpected vinylogous Wolff rearrangement provided access to the C9 quaternary stereocenter and formed the basis for a synthetic route to C12 normethyl pleuromutilins.
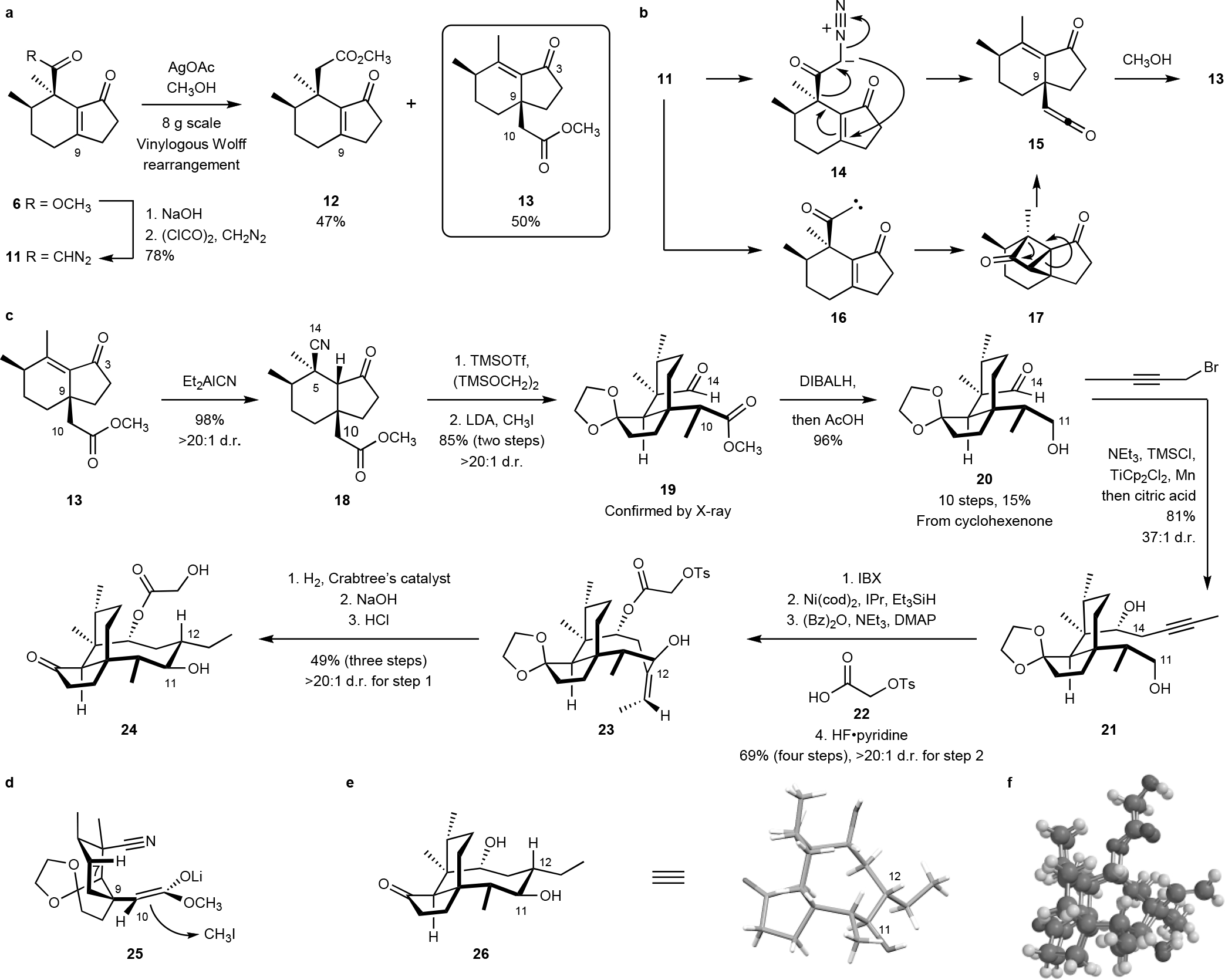
a. Arndt–Eistert homologation of the α-diazoketone 11 provided the homologation product 12 and the rearrangement product 13. b. Potential mechanistic pathways for the rearrangement may involve a concerted 2,3-rearrangement of the α-diazoketone 14 or rearrangement of the ketocarbene 16. c. Synthesis of the C12 normethyl pleuromutilin derivative 24. d. Proposed orientation of the (Z)-enolate 25. e. X-ray structure of the mutilin derivative 26. f. MMFF (Merck molecular force field) overlay of 1 and 24.
