Scheme 1.
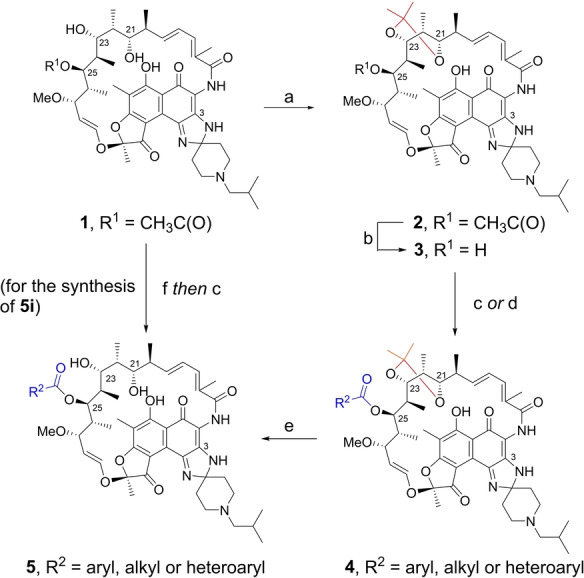
Synthesis of 25‐O‐acyl rifabutin analogs: a) 2,2‐dimethoxypropane, CSA, acetone, room temperature, 2 h, 68 %; b) K2CO3, MeOH, 50 °C, 48 h, 59 %; c) RC(O)OC(O)R, DMAP, 1,2‐dichloroethane, room temperature or 50 °C, 72–96 h; d) RCOOH, pivaloyl chloride, triethylamine, DMAP, DCM, 0 °C to room temperature, 4 h; e) CSA, MeOH, room temperature, 0.5 h; f) NaOH, ZnCl2, MeOH, room temperature, overnight, 71 %. For 5 a–5 h and 5 j–5 o, the yields were 7–72 % over two steps (c or d, and then e); For 5 i, the yield was 6 % with procedure c from 25‐O‐desacetyl rifabutin 6. CSA=camphorsulfonic acid.
