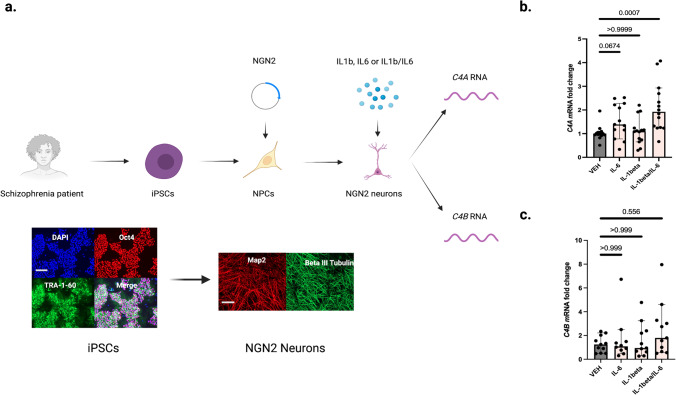
Fig. 2. Disease-associated cytokines increase neuronal C4A mRNA expression in a patient derived schizophrenia model.
a Overview of in vitro experiments in which induced pluripotent stem cells (iPSCs) derived from four chronic patients (all white males, 28- years old, 44-years old, 55-years old, and 58-years old, respectively) were differentiated into cortical excitatory neurons. Representative quality control immunocytochemistry images display octamer binding transcription factor 4 (POU domain, class 5, transcription factor 1) and TRA-1–60, as well as nuclear staining with DAPI for iPSCs that were used to derive cortical excitatory neurons (here stained for MAP2 and Beta III Tubulin). Scale bars for representative images:100 uM. b qPCR was used to measure relative mRNA expression of C4A. The relative C4A mRNA expression increased significantly after pre-treatment for 24 h with the interleukin (IL)−1beta and IL-6 combination (median fold change = 1.9, 95% confidence interval [CI] = 1.5–2.7) compared to vehicle (VEH) stimulated neuronal cultures (median = 1.0, CI = 0.86–1.2, adjusted P = 0.0007), while the increase in relative mRNA expression was not significant after IL-1beta (median = 1.1, CI = 0.79–1.5, adjusted P = 0.999) or IL-6 stimulation (median = 1.4, CI = 1.1–2.0 adjusted P = 0.067). n = 14 biologically independent derivations (datapoints) per condition (n = 13 for IL-6 exposure condition). qPCR reactions were done in duplicate and averaged. The experiment was repeated three times. As a sensitivity analysis, we also analyzed the data using a generalized linear mixed effect model (robust estimation to handle violations of model assumption and adjusting for multiple testing using sequential Sidak). Target: CSF C4A concentration, fixed effects: experimental condition (VEH, IL-1beta, IL-6, IL1-beta/IL-6), cell donor (n = 4 categories), and experimental round (n = 3 categories). Significant effects were observed for experimental condition (F = 8.9; P = 2.2 × 10−4; estimated means: VEH = 0.78 [CI = 0.44–1.01], IL1-beta=0.78 [CI = 0.39–1.17], IL-6 = 1.23 [CI = 0.84–1.62], IL-1beta/IL-6 = 1.78 [CI = 1.36–2.20], fixed effect coefficient for VEH condition [IL-1beta/IL-6 as reference group] = −1.06 [CI = −1.53 to −0.58]; P = 4.6 × 10−5) and for donor (F = 4.6; P = 0.007). c Compared to the vehicle (VEH) stimulated neurons (median fold change = 1.2, CI = 0.82–1.7) the relative C4B mRNA expression was not significantly increased after IL-1beta stimulation (median fold change = 0.94, CI = 0.65–2.6, adjusted P = 0.999), IL-6 stimulation (median = 1.1, CI = 0.33–3.0, adjusted P = 0.999), or IL-1beta/IL-6 stimulation (median fold change = 1.8, CI = 1.0-4.0, adjusted P = 0.556). n = 11 biologically independent derivations (datapoints) per condition (n = 10 for IL-6 exposure condition). qPCR reactions were done in duplicate and averaged. ∆Ct values were obtained by comparison to Ct values of a housekeeping gene, and fold changes (2 − ∆∆Ct) were then generated by normalization to the average ∆Ct value for VEH treated wells per plate (see Methods). Similar data were observed across three independent experiments. Kruskal–Wallis H tests followed by post-hoc tests. Significance was set to P < 0.05. All reported p-values are two-sided. Figure 2a was created with BioRender.com. Source data for graphs in Fig. 2b, c are provided in the Source Data file.