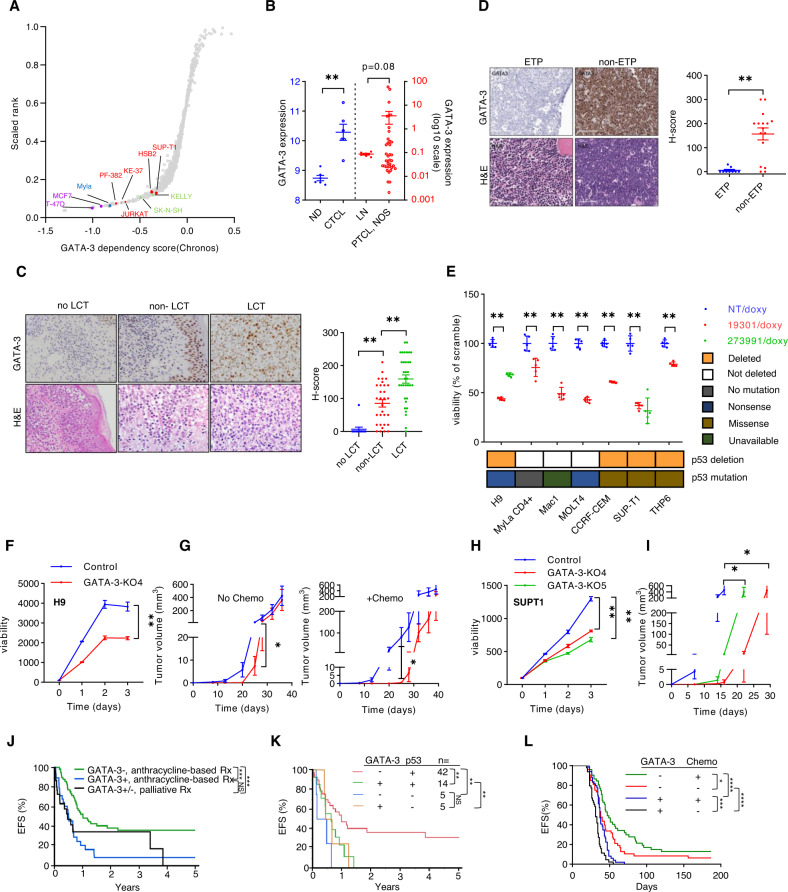
Fig. 1. GATA-3 dependency in T-cell lymphoproliferative neoplasms.
A A scatter plot showing relative dependency on GATA-3 in T-cell acute lymphoblastic leukemia (T-ALL) cell lines (n = 5, in red), a mature T-cell lymphoma cell line (n = 1, in blue), and 1048 additional cell lines, including breast cancer (in purple) and neuroblastoma (in green) cell lines, for which GATA-3 is a known dependency. The Y-axis shows the GATA-3 dependency rank and the X-axis shows the GATA-3 dependency score from Chronos (21Q3) for each individual cell line. B Expression of GATA-3 transcripts were collected from three microarray datasets. Two datasets from Normal donors (ND) and Sézary syndrome (CTCL) patient samples are shown at left (in Gene Expression Omnibus database, accession number: GSE131738 and GSE39041), and a dataset including reactive lymph nodes (LN) and PTCL, NOS biopsies is shown at right (accession number: GSE36172). C GATA-3 immunohistochemistry and hematoxylin and eosin (H&E) staining was performed in skin biopsies obtained from CTCL patients with limited-stage (patch/plaque) disease that never developed large cell transformation with clinical follow-up (no LCT, n = 12), and in paired biopsies obtained from patients before (non-LCT, n = 31) and after LCT (LCT, n = 34). Representative examples are shown (at left) and the data summarized (at right). D GATA-3 immunohistochemistry was similarly performed in a cohort of T-ALL biopsies, including early thymocyte progenitor (ETP, n = 9) and non-ETP specimens (n = 16). Representative images are shown (at left) and the data summarized (at right). E CTCL and T-ALL cell lines were transduced with doxycycline-inducible constructs expressing GATA-3 (19301, 273991) or non-targeting (NT) shRNA. Relative cell viability was determined 7 days after GATA-3 knockdown. As comparable results were achieved with two independent shRNA in H9 and SUPT-1 cells, the remaining cell lines were transduced with the GATA-3 targeting shRNA (19301, in red) associated with the most significant GATA-3 knockdown. P53 deletions and mutations are prevalent in these cell lines, and are indicated below. F CRISPR/cas9-mediated GATA-3 knockout (KO) was achieved in H9 cells (KO4), and cell proliferation and viability determined by RealTime-Glo. Y-axis demonstrates luminescence intensity. G NSG mice were injected subcutaneously with control and GATA-3 KO H9 cells, and mice treated with cyclophosphamide and vincristine (or vehicle control) on days 24 and 31 (n = 10). Tumor volumes, stratified by treatment, are shown. H, I Cell proliferation and viability was similarly determined in two independent GATA-3 KO SUP-T1 subclones (H) and tumor volumes (I) measured in NSG mice bearing control (n = 8) and GATA-3 KO xenografts (n = 6). Y-axis demonstrates luminescence intensity. J, K A cohort of PTCL, NOS patients was stratified by GATA-3 expression and treatments received, and event-free survival (EFS) examined (J). Loss of the p53 locus (17p13.1) was determined by FISH in the subset of cases for which tissue had not been exhausted, and EFS similarly examined (K). l Event-free survival (EFS) from Splenocytes from lymphoma-bearing GATA-3fl/fl (n = 9) and GATA-3+/+ or fl/+ (n = 10) SNF5fl/fl, CD4-Cre mice were adoptively transferred to B6 recipients (n = 4–5/biologic replicate) and were treated with cyclophosphamide and vincristine (or vehicle control) every 7 days for 2–3 weeks, and EFS determined. Data are represented as mean ± s.e.m. (standard error of the mean). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.