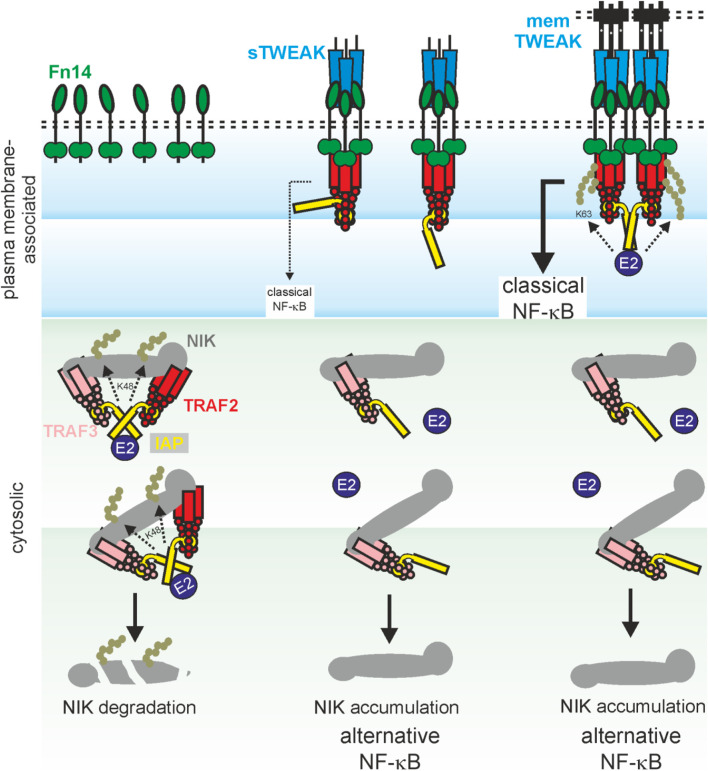
FIGURE 1.
Model of TWEAK-induced NF-κB signaling. Dependent on the interplay of sTWEAK, memTWEAK, Fn14, TRAF-cIAP1/2 and TRAF3-cIAP1/2-NIK complexes, three different states of NFκB signaling can be distinguished: i) In the absence of TWEAK, TRAF3-cIAP1/2-NIK complexes interact with TRAF2-cIAP1/2 complexes in the cytoplasm. This results in cIAP1/2 transactivation and by help of E2 proteins to K48 ubiquitination of NIK by the cIAPs, proteasomal degradation of NIK and thus in constitutive inhibition of the alternative NF-κB pathway (left panel). ii) In the presence of sTWEAK, ligated trimeric Fn14 complexes are formed which recruit a single TRAF2-cIAP1/2 complex and reduces so the availability of the latter for NIK degradation. As a consequence, there is reduced NIK degradation leading to accumulation of NIK and eventually to signal-induced alternative NF-κB signaling. Since the Fn14-bound single TRAF2-cIAP1 and TRAF2-cIAP2 complexes remain largely inactive due to missing cIAP1/2 transactivation, there is no/poor classical NFκB signaling (middle panel). iii) In the presence of memTWEAK, ligated trimeric Fn14 complexes are formed, too but due to their high concentrations in the cell-to-cell contact zone these complexes cluster secondarily leading to the recruitment of several TRAF2-cIAP1/2 complexes to the Fn14 clusters. Again, this reduces the availability of TRAF2-cIAPs complexes for NIK degradation and triggers the alternative NF-κB pathway. However, since the Fn14-clusters bind several TRAF2-cIAP1/2 complexes, transactivation of cIAPs becomes possible leading eventually in classical NFκB signaling (right panel).