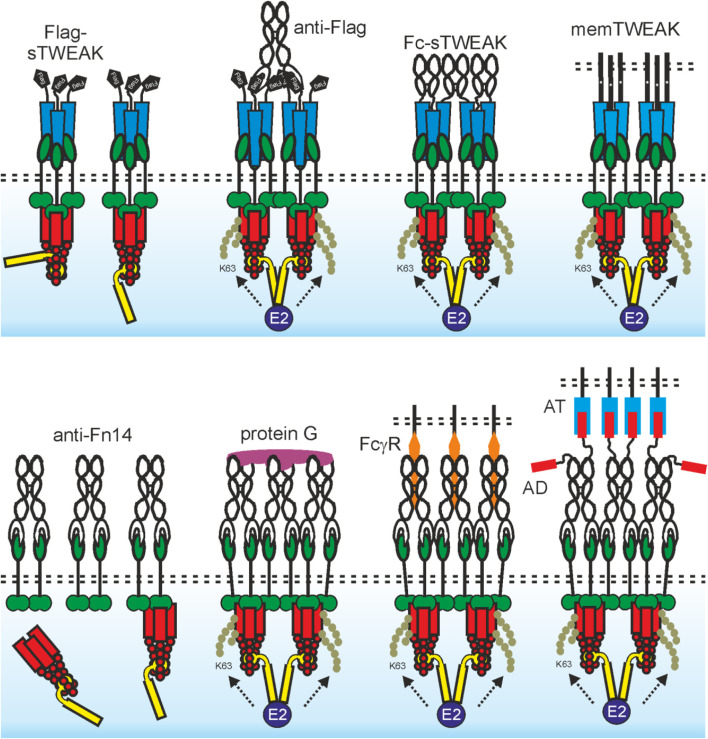
FIGURE 2.
The agonistic quality of TWEAK trimers and anti-Fn14 antibodies is determined by oligomerization and membrane-association. Upper panel: An sTWEAK trimer recruits three Fn14 molecules. The Fn14 trimers assembled by sTWEAK in turn recruit complexes of a TRAF2 trimer with one cIAP1 or cIAP2 molecule. This results in reduced availability of these molecules for other binding partners but does not trigger efficient cIAP transactivation. If the proximity of two (or more) of these sTWEAK-associated Fn14 trimers is enforced, e.g., by use of antibody crosslinking (e.g., Flag-tagged sTWEAK with anti-Flag antibody) or by genetic fusion with an oligomerization domain (e.g., Fc domain), this results in close neighborhood of two (or more) TRAF2-cIAP1/2 complexes and thus in cIAP transactivation and classical NF-κB signaling (see also Figure 1). Due to the high concentrations of trimeric Fn14 complexes in the cell-to-cell contact zone with memTWEAK expressing cells, there is spontaneous clustering of the Fn14 complexes and again cIAP transactivation due to the tight neighborhood of TRAF2-cIAP1/2 complexes. Lower panel: An anti-Fn14 antibody recruits two Fn14 molecules. The anti-Fn14-bound Fn14 dimers recruit one TRAF2-cIAP1/2 complex but less efficient than an sTWEAK trimer. Thus, there is some reduction in the cytosolic availability of TRAF2-cIAPs and therefore alternative NF-κB signaling despite weaker as with sTWEAK (see upper panel and Figure 1). If the linkage of three (or more) of these anti-Fn14-associated Fn14 dimers is enforced, e.g. by protein G crosslinking this also induces neighborhood of several TRAF2-cIAP1/2 complexes and thus cIAP transactivation and classical NF-κB signaling. Due to the high concentrations of dimeric Fn14 complexes in the cell-to-cell contact zone with cells decorated with FcγR-bound anti-Fn14 antibodies (or anchoring target (AT)-bound anti-Fn14 antibody fusion proteins with an anchoring domain (AD), there is again spontaneous clustering of the Fn14 complexes and cIAP transactivation.