Abstract
The large number of microbes found in the gut are involved in various critical biological processes in the human body and have dynamic and complex interactions with the immune system. Disruptions in the host’s gut microbiota and the metabolites produced during fermentation promote the development of intestinal inflammation and colorectal cancer (CRC). Toll-like receptors (TLRs) recognize specific microbial-associated molecular patterns specific to microorganisms whose signaling is involved in maintaining intestinal homeostasis or, under certain conditions, mediating dysbiosis-associated intestinal inflammation. The signaling pathways of TLRs are described first, followed by a discussion of the interrelationship between gut microbes and TLRs, including the activation of TLRs by gut microbes and the effect of TLRs on the distribution of gut microbiota, particularly the role of microbes in colorectal carcinogenesis via TLRs. Finally, we discuss the potential roles of various TLRs in colorectal cancer.
Keywords: Toll-like receptors, gut microbes, colorectal cancer, NF-κB, pattern recognition receptor
Background
Colorectal cancer (CRC) is one of the most common cancers, ranking third in malignancy diagnoses and second in cancer deaths globally, and tends to increase at younger ages (before age 50).1,2 CRC is a multifactorial disease, and it is considered that inheritance, age, environment, lifestyle and other factors influence its occurrence.3,4 Evidence that high-fat diets, high-protein diets, and consumption of red and processed meats are high-risk factors for cancer, while a high-fiber diet significantly reduces the incidence of CRC.3,5 As high-throughput sequencing technology advances and more research is conducted, intestinal flora has become a focal point for CRC causation and prevention studies.
Many microbes, including bacteria, viruses, archaea, and fungi, colonize the human gut, with bacteria being the most common. The gastrointestinal ecosystem is formed at birth and changes continuously throughout life, with the microbial composition of the gut of healthy people being highly specific and in dynamic equilibrium.6-9 The gastrointestinal tract is the most colonized part of the human body by microbes, accounting for more than 70% of the total.10 Microbes play an important role in the gastrointestinal tract that affects not only metabolism and nutrient absorption, but also the human immune system and the development of colorectal cancer.11-14 Intestinal microbes influence colorectal cancer progression by producing virulence factors, metabolites, and effects on host inflammation and immunity.15,16
Toll-like receptors (TLRs) are members of the pattern recognition receptor family that recognize microorganism-specific molecular patterns. Bacterial antigens, such as peptidoglycan and lipopolysaccharide (LPS), are involved in the TLR signaling pathway.17 The dynamic nature of the gut microbial community necessitates host tolerance and monitoring to prevent pathogenic colonization and to protect symbiotic microbes. Any imbalance in the microbiome can disrupt TLR signaling pathways, resulting in uncontrolled inflammation and diseases like inflammatory bowel disease (IBD), which can eventually lead to CRC.18,19
TLR-mediated interaction between the microbiota and the intestinal mucosa is essential in maintaining dynamic intestinal homeostasis. TLR is increasingly implicated in tumor progression, particularly in microbiota-associated CRC.20-22 Specific TLRs overexpressed in tumor cells can inhibit growth, whereas other TLRs aid tumorigenesis and progression. When these TLRs are activated, they can cause inflammation, tumor cell proliferation, immune evasion, local invasion, and distant metastasis.23 In this review, we focus on the TLR signaling pathway, the interaction between the gut microbiota and TLR, and their role in the development of CRC.
TLR Family and Its Pathways
TLR is a type 1 transmembrane receptor expressed on epithelial and lamina propria cells. It contributes to host cell recognition and response to microbial pathogens by recognizing multiple pathogen-associated molecular patterns and damage-associated molecular patterns.14,24-26 Ten TLRs have been identified in humans to date, and the TLR family is divided into two sub-types based on their location in the cell. TLRs 1, 2, 4, 5, 6, and 10 are expressed on the cell surface and recognize extracellular microorganisms; TLRs 3, 7, 8, and 9 are thought to detect the presence of viral particles.27,28
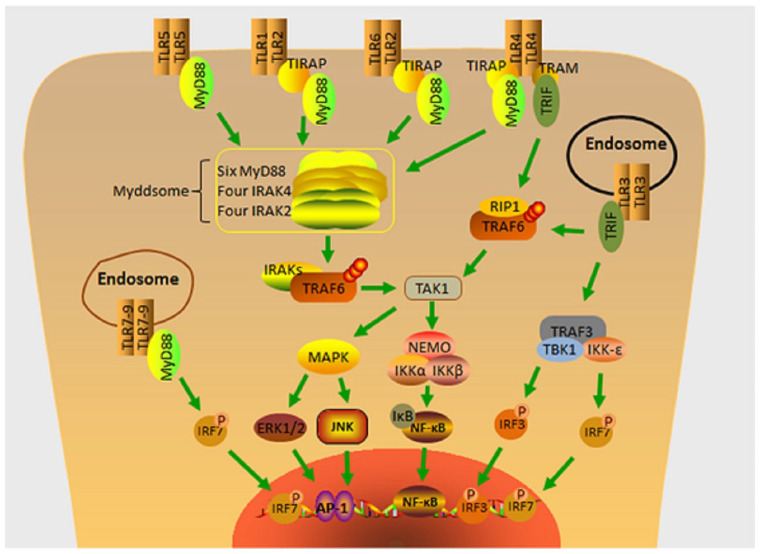
TLRs on cell membranes or nuclear endosomes recognize ligands such as structures, flagellin, and single- and double-stranded RNAs from Gram-positive and Gram-negative bacteria and induce receptor dimerization, with most TLRs forming homodimers except for TLR2, which includes a heterodimer with TLR1 or TLR6,29 allowing intracellular TIRs to interact with different intracellular TIR-containing adapters. There are five TIR-containing adapters, including myeloid differentiation primary response protein 88 (MyD88) and TIR domain-containing adaptor-inducing IFN-β (TRIF; also known as TICAM-1), MyD88 adaptor-like protein (TIRAP/Mal), TRIF-related adaptor molecule (TRAM), Sterile-alpha and Armadillo motif-containing protein. TLR signaling is broadly separated into two different pathways using other adaptor molecules, MyD88 and TRIF. The signaling pathway generated by the recruitment of MyD88 articulators is called the MyD88-dependent pathway, while the pathway induced by the recruitment of TRIF is called the MyD88-independent pathway or the TRIF pathway.30-32
The MyD88-dependent pathway is involved in the early initiation of NF-κB, and all TLRs but TLR3, which only signals via TRIF, have been shown to activate the course. MyD88 contains an N-terminal dead domain (DD), an intermediate domain, and a C-terminal TIR domain; in most cases, MyD88 recruits directly to TLR activation, while TLR2 and TLR4 require TIRAP/Mal involvement.33,34 TLR-bound MyD88 recruits and activates other members of the IRAK family, like IRAK-1 and IRAK-2, through their respective DD interactions with IL-1R-associated kinase (IRAK)-4 to form Myddsome.35,36 IRAK-1 then undergoes autophosphorylation, which interacts with tumor necrosis factor (TNF)-α receptor-associated factor 6 (TRAF6). IRAK-1 and TRAF6 are subsequently detached from the complex, and TRAF6 functions as an E3 ubiquitin protein ligase, catalyzing the forming of lysine 63 (K63)-linked polyubiquitin chains between TRAF6 itself and the E2 ubiquitin ligase complex consisting of Ubc13 and Uev1A. As a scaffold, this polyubiquitin chain recruits TGF-β-activated kinase 1 (TAK1) with its bound proteins TAB1, TAB2, and TAB3, leading to phosphorylation and activation of IKKα/β. Then, the IKK complex consisting of IKK-α, IKK-β, and the nuclear factor-kappaB (NF-κB) essential modulator (NEMO, also known as IKKγ) phosphorylates the inhibitor of NF-κB (IκB). This phosphorylation leads to the degradation of IκB, and the release of NF-κB into the nucleus, and this phosphorylation results in the IκB degradation. NF-κB is released into the nucleus and later synthesized into pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, which are inflammatory key media.36-38 In addition, TAK1 affects translation by triggering the phosphorylation of mitogen-activated protein kinases (MAPKs), activating the MAPKs Erk1, Erk2, p38, and Jnk, which in turn activates several transcription factors, including AP-1.39-41
TLR4 is unique among TLRs because it can conduct signals through both the MyD88-dependent pathway and the TRIF pathway; the two pathways have different dynamics, and TLR4 initiates recruitment of TIRAP and MyD88. TLR4 activation of TRIF necessitates the involvement of TRAM.42 TLR3 and TLR4 activate TRIF, which binds to TRAF3 and TRAF6 via the presence of their N-terminal partial TRAF-binding groups. TRIF activates TAK1 upon interaction with TRAF6 via RIP1, which then starts NF-κB in a way similar to the MyD88-dependent pathway. TRAF3 triggers TANK-binding kinase 1 and IKK-i (also known as IKK-ɛ), phosphorylates IFN regulatory factor-3 (IRF3) and IRF7, and activates the type I interferon (IFN) signaling pathway43,44 (Figure 1).
Figure 1.
TLR signaling pathways. TLR pathways are divided into two types: those mediated by MyD88 adaptor proteins and those mediated by MyD88-independent adaptor proteins, also known as the TRIF pathway. All TLRs activate the MyD88-dependent pathway except for TLR3, which exclusively signals through TRIF. Activated TLRs attract MyD88, which subsequently starts targets further down the chain. NF-κB, IRF3 and IRF7, as well as transcription factor activation and the generation of inflammatory cytokines, are all activated by TLR4, which leads to inflammation, immunological modulation, survival, proliferation, and cancer via the TRIF and MyD88 pathways. IRAK, IL-1R-associated kinase; IRF3, IFN regulatory factor; MAPK, mitogen-activated protein kinases; MyD88, myeloid differentiation primary response protein 88; NEMO, NF-κB essential modulator; NF-κB, nuclear factor-kappaB; TIRAP, TIR domain-containing adaptor-inducing IFN-β, MyD88 adaptor-like protein; TLR, Toll-like receptors; TRAF, tumor necrosis factor (TNF)-α receptor-associated factor 6; TRIF, TIR domain-containing adaptor-inducing IFN-β.
TLR and Gut Microbial Homeostasis
The gastrointestinal tract is microbially colonized shortly after birth, and a simple bacterial community gradually develops into a complex ecosystem that then begins to show a symbiotic relationship with the host.45 Without perturbations, the gut microbial community oscillates around a stable ecological state, displaying dynamic balance. The gut microbiota is typically highly resilient to disruption, allowing the host to retain key species over time, and the microbiome remains relatively stable in adulthood46; however, the composition of the resident microbiota may be altered by environmental factors such as diet and antibiotic use,47,48 there are no single species that is universally found in all humans.49 The gut microbiome provides numerous benefits to the host, including the breakdown of indigestible foods, the provision of energy to colonic epithelial cells, and the provision of a barrier to invading pathogens; they also have significant effects on many host systems, particularly the development of the gut and immune system. The microbiota are the most important source of microbial stimulation in the intestine and are required for developing the intestinal immune system.50
The microbiota, the persistent presence of gut microbes, that modulate TLR expression and activation, strongly influences TLR expression as an innate immune sensor. Changes in gut microbiota composition may differentially modulate the responsiveness of mucosal TLRs, subverting the immune response dominated by a pro-inflammatory phenotype.51 As we mentioned above, the location of TLRs is crucial for their function, and the gut microbiota can influence the area of TLRs; it was found that TLR1, TLR2, TLR5 and TLR9 were found to be up-regulated in specific pathogen-free mice compared with germ-free mice,52-54 while TLR1, TLR2, TLR4 and TLR5 were most highly expressed in the colon with a high microbial burden.55,56
The symbiotic gut microbiota is essential in host development and inflammation. Luminal microbial populations are determined by a combination of environmental and host genetic factors, particularly the innate immune system. The role of the natural immune system in defense against pathogenic microbial infections is critical, and its importance in regulating the gut microbiota is becoming increasingly recognized.57 The TLR is the intestinal epithelial barrier, the interface between the microbiome and the immune system, and cannot be overlooked in shaping the gut microbiome.51 TLR5 (a cell surface receptor that recognizes bacterial flagellin) expression on IECs, for example, regulates the composition and location of the intestinal microbiota to prevent diseases associated with intestinal inflammation.58,59 TLR5 deficiency in mouse intestinal epithelial cells altered the intestinal microbiota, resulting in low inflammation, metabolic syndrome, and colitis.59,60 The TLR trigger found in IEC is proposed to maintain immune stress on symbiotic flora, limiting symbiotic and pathogenic bacteria colonization and translocation.61
Gut Microbiota Affects CRC Progression Through TLR-Related Pathways
TLR’s role in CRC is becoming clear as research into the role of gut microbiota continues. TLR’s effect on cancer progression has not been conclusively established, and continued positive TLR expression throughout the course of CRC lesions suggests that TLR may promote CRC progression,62,63 several research studies have also indicated that TLR has a dual role in cancer progression.64 TLR agonists are widely used in treating infectious diseases and as adjuvants in cancer treatment because of their role in specific and non-specific immunity.65
TLR Mediates Inflammation and Increases Cancer Cell Stemness via NF-κB to Promote CRC Progression
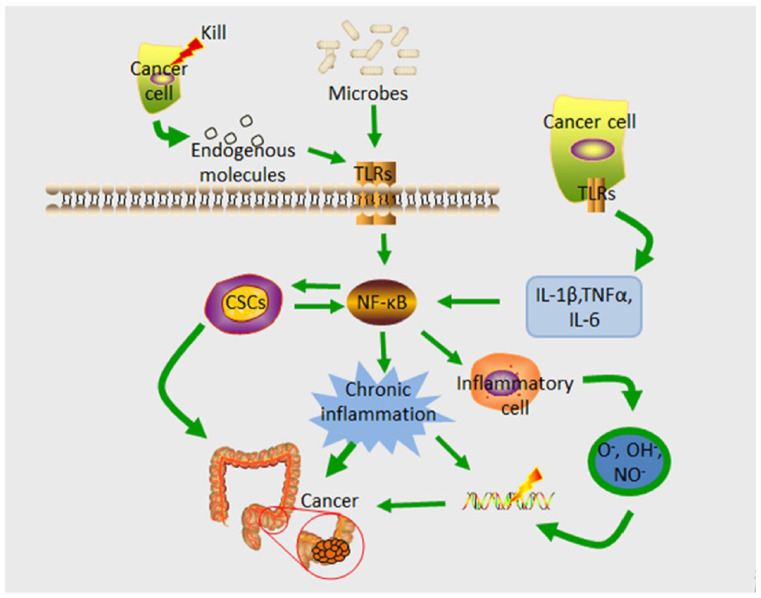
Inflammation is an essential factor in tumorigenesis; it is not only one of the factors that promote cancer progression, but it is also a significant contributor to tumor formation.66 Chronic inflammation is well known to contribute to the development of CRC,67-69 and the long-term use of anti-inflammatory drugs dramatically lowers the risk of CRC.70 TLR senses and recognizes the gut microbiota and plays a vital role in maintaining gut homeostasis, controlling immune responses, and shaping the microbiome; dysfunctional expression and function are linked to the onset of inflammation and CRC.71 TLRs activate the NF-κB signaling pathway when stimulated by TLR endogenous ligands released by oncogenic microbes and cells killed during cancer treatment.72,73 TLR activation in tumor cells upregulates the manifestation of several inflammatory cytokines, IL-1β, TNFα, and IL-6. These cytokines are released into the tumor microenvironment, where they activate NF-κB, resulting in sustained inflammatory cell responses and the development of chronic inflammation.74 Enhancement of the NF-κB signaling pathway is a primary pro-tumor function of TLR. NF-κB triggers the transcription of many pro-inflammatory genes and is a vital mediator of both acute and chronic inflammatory responses. Chronic inflammation modifies the tumor microenvironment, which serves as a scaffold and barrier for tumor growth and promotes tumor formation and development.75,76 The inflammatory microenvironment can cause CRC by increasing the mutation rate and allowing mutant cells to proliferate. Inflammatory cells that are activated can produce reactive oxygen species (ROS) and reactive nitrogen intermediates, which can cause DNA damage and genomic instability.66
Furthermore, NF-κB-mediated inflammation increased the stemness of cancer cells, and cancer stem cells (CSCs) constitutively showed elevated NF-κB activation, which, in turn, enhanced their stemness. These interactions result in a positive feedback loop that magnifies cancer cell inflammation and stemness, increasing the number of CSCs in the tumor. As a result of their role in cancer transfer, tolerance to treatment, and recurrence after treatment, these cells may contribute to malignancy and reduce the outcome of successful cancer therapy77 (Figure 2).
Figure 2.
TLR via NF-κB to promote CRC progression. An important mediator of both acute and chronic inflammatory responses, TLR activation on cells stimulated by endogenous ligands activates the NF-κB signaling pathway, triggering the transcription of many pro-inflammatory genes. In addition to enhancing the proliferative capacity of mutant cells, this results in DNA damage, which increases mutation rates. In addition, inflammation caused by NF-kB contributes to an increase in the stemness of cancer cells that already have an elevated level of NF-kB expression. This, in turn, enhances the cancer cells’ stemness, which in turn increases the number of CSCs in the tumor, which in turn contributes to cancer metastasis, treatment resistance, and recurrence after treatment. CSCs, cancer stem cells; IRF3, IFN regulatory factor; NF-κB, nuclear factor-kappaB; TLR, Toll-like receptors; colorectal cancer; TNFα, tumor necrosis factor; TRIF, TIR domain-containing adaptor-inducing IFN-β.
TLR Promotes CRC Progression by Mediating Aberrant Cell Proliferation and Anti-apoptotic Effects
Cancer is distinguished by resistance to cell death and the activation of invasion and metastasis,78 TLR signaling is tightly linked to abnormal cell multiplication and death resistance, as well as increased tumor cell invasion and metastasis via matrix metalloproteinases and integrin regulation.79 Pro-inflammatory signals typically reduce the components of the adaptive immune response. TLR influences inflammatory homeostasis and suppresses antitumor immunity, causing this reaction to shifting from antitumor to pro-tumor.23 NF-κB is closely associated with anti-apoptotic pathways that control the antiapoptotic gene expressed and also limits the activities of pro-apoptotic pathways such as JNK.80 In CRC, TLR-induced NF-κB stimulation was shown to promote tumor cell survival.81 Tumor-derived factors such as IL-6, IL-10, VEGF, and TGF-β may bias immune response induction and lead to tumor resistance in the tumor microenvironment,82 TLR promotes the release of these cytokines.83 TLR4 was found to be overexpressed in inflammation-associated colorectal tumors in humans and mice. In contrast, TLR4-deficient mice were significantly colorectal cancer-protected, indicating that TLRs on tumor cells either directly or indirectly contribute to tumor development.84 Furthermore, a study in the MC26 mouse colon cancer cell line discovered that TLR4 stimulation decreased both tumor cell sensitivity to CTL attack and the function of T cells and natural killer (NK) cells and that blocking TLR4 signaling delayed tumor growth and prolonged the survival of tumor-bearing mice.85
TLR-Mediated Antitumor Effects
Because TLRs function as signaling agents in immune surveillance, the use of TLRs against tumors is becoming more researched. TLRs influence tumor progression via multiple pathways and numerous studies have revealed that TLRs and their ligands have antitumor effects by inducing apoptosis/necrosis in tumor cells or activating immune cells.86 TLR-activated DCs can mediate antitumor responses through antigen presentation and effects on T cells and direct cytotoxic effects on tumor cells. TLR agonists can also target CD4 + and CD8 + cells, improve their survival, increase cytokine secretion, and enhance their cytotoxic and antitumor effects.87,88 TLR ligands can also be used as immunostimulatory molecules to boost the immune system’s anti-cancer therapy, and TLRs have been used in numerous preclinical and clinical studies in this regard.89 TLR agonists promote dendritic cell maturation and the production of pro-inflammatory cytokines such as IFN, which stimulates the adaptive immune system.90 TLR9 has been studied in CRC because it has been shown to have a potential protective effect against the malignant transformation of the colorectal mucosa.91 In phase I clinical trials, TLR 9 agonists (for example, MGN1703) was shown to have immune activating and antitumor efficacy.92 Although the primary endpoint of improved overall survival/progression-free survival was not met in the intention-to-treat group in this study, MGN1703 was evaluated as maintenance therapy in patients with metastatic colorectal cancer and disease control after chemotherapy in phase II clinical trials.93 However, its potential role in the maintenance treatment of metastatic CRC following tumor response induction with an effective first-line regimen prompted a phase III trial of subcutaneous MGN1703 as maintenance therapy versus standard maintenance regimens for patients with metastatic CRC. They have achieved tumor shrinkage with induction therapy (NCT02077868). In addition, other TLR9 agonists such as tilsotolimod (IMO-2125), SD-101, and so on, are under investigation.94 Interactions between tumor cells and immune cells in the tumor’s microenvironment promote the aberrant immune enhancement and antitumor effects in the TLRs signaling pathway; additionally, TLRs can inhibit tumor progression by inducing programmed tumor cell death.95
Involvement of TLRs in CRC
TLR2/4
TLR2 and TLR4 have received the most attention. TLR2 is the primary signaling receptor for Gram-positive bacteria’s cell wall components peptidoglycan and lipoprotein acid, whereas TLR4 is the primary signaling receptor for Gram-negative bacteria’s cell wall component LPS (Table1).96,97 TLR2 and TLR4 have been linked to infections, inflammation, allergic diseases, and carcinogenesis.81,98,99 TLRs are strongly linked to microbiota-induced colitis-associated carcinoma (CAC), and severe chronic colitis may be linked to the direct progression of CRC from adenoma to invasive carcinoma.100 There is no uniform conclusion on the function of TLR2 and TLR4 in CRC.
Table1.
TLRs and their associated ligands.
| TLR | Localization | Gene and its location | Ligands (PAMPs and DAMPs) | Origin of ligands |
|---|---|---|---|---|
| TLR2 | Plasma membrane | 4q31.3 | Peptidoglycan, lipoteichoic acid, lipoproteins, lipoarabinomannan, phenol-soluble modulin, glycoinositolphospholipids, glycolipids, porins, zymosan, atypical lipopolysaccharide, heat shock protein70(Hsp70), eosinophil-derived neurotoxin acts, an alarmin | Gram-positive bacteria, Mycobacteria, Fungi, Host-derived DAMPs |
| TLR3 | Endolysosome | 4q35.1 | Double-stranded RNA (dsRNA) | Viruses |
| TLR4 | Plasma membrane | 9q33.1 | Atypical lipopolysaccharide: Gram-negative bacteria, taxol, fusion protein, envelope proteins, high mobility group box 1 protein, Hsp60, Hsp70, Hsp22, Hsp96, Type III repeat extra domain A of fibronectin, oligosaccharides of hyaluronic acids, polysaccharide fragments of heparin sulfate, fibrinogen, saturated fatty-acids | Gram-negative bacteria, Plant, Respiratory syncytial virus (RSV), Mouse mammary tumor virus (MMTV), Host-derived DAMPs |
| TLR9 | Endolysosome | 3p21.2 | CpG oligodeoxyneucleotide, hemozoin pigment, Histones | Bacteria and viruses (HSV), Malaria, Host-derived DAMPs |
TLR, Toll-like receptors.
References: Kawai and Akira101; Basith et al102; Botos et al103; Chang104; Ding et al105; Kumar and Barrett106; Medvedev107; Ohto108; Sameer and Nissar18; Sandor and Buc.109
TLR2, which is highly expressed in CAC, has a standard function in tumor proliferation in CAC and sCRC, according to a study based on TLR2 knockout mice, and TLR2 knockdown inhibits CAC and sCRC growth, reduces tumor severity, and decreases tumor proliferation.110 TLR2-deficient mice developed more extensive and more colonic tumors, implying that TLR2 protects against CRC and that TLR2 deficiency causes CAC and early intestinal tumors.111 Another study found that TLR2 expression was significantly upregulated at the protein and mRNA levels in CRC patients. Still, there was no correlation between TLR2 expression and CRC staging, which is consistent with previous findings. However, TLR2 promotes CRC multiplication, migration, and invasion, and TLR2 inhibition directly inhibits CRC growth1.112
TLR4 recognizes a variety of pathogens, and its expression varies in different intestinal regions and is heavily influenced by the region’s microbial composition.113 TLR4 promotes the development of colorectal tumors in chronic colitis, and microorganisms abundant in the intestine may activate TLR4 via their antigens (e.g., antigenic lipopolysaccharides of Gram-negative bacteria), thereby inducing the growth of CRCs.84 TLR4 is thought to be expressed and functionally active on human CRCs, and it may help CRCs escape the immune system by activating immunosuppressive factors and apoptosis resistance.114,115 TLR4/MyD88 signaling promotes CRC progression by promoting hepatic metastasis. Silencing TLR4 / MyD88 signaling in tumor cells reduces tumor development. It can increase mouse survival time after subcutaneous tumor injection.116 TLR4 and MyD88 immunohistochemistry in the normal colonic mucosa and adenomas. CRC revealed that elevated levels of TLR4 and MyD88 expression are associated with liver metastasis and are independent predictors of poor prognosis in CRC patients. TLR4/MyD88 signals were found to be responsible for CRC tumorigenesis in colitis-associated cancers and sporadic CRC.117 TLR4 mRNA expression in CRC tissues has not been shown to be significantly different from that in normal tissues, and TLR4 expression may not be invol.118
TLR3
The TLR3 gene is found on the long arm of chromosome 4, and intestinal epithelial cells of normal mucosa express TLR3 constitutively,119 which recognizes live bacteria’s double-stranded RNA and is a crucial component member of the innate immune system.120 TLR3 is protective against intestinal injury. It was found that upon TLR3 activation, it initiates a series of signals that trigger the production of interferon regulatory factor 3 and other cytokines and ultimately activate NF-κβ, which stimulates the adaptive immune system.119 According to a large case-control study, TLR3 significantly interacts with colorectal cancer. Genetic variants in TLR3 may influence the development of colon cancer and the impact on survival after colon cancer diagnosis.121 Previous research has shown that TLR3 agonists activate tumor-specific immune responses in mice and patients. Research into the safety and therapeutic potential of TLR3 agonists is ongoing, and combination therapy with TLR3 agonists may be a treatment option for colorectal cancer.122
TLR9
The TLR9 gene is found on chromosome 3 and comprises two exons that encode 1032 amino acids. It is mainly found in intracellular vesicles (e.g., endoplasmic reticulum, lysosomes, endosomes, and lysosomes),123 and detects unmethylated CpG groups in bacterial DNA, activating the TLR9 signaling pathway, which initiates a type 1 T helper cell immune response and promotes B cell proliferation, protecting the host from invading outside microbes.124 TLR9 signaling is involved in colon carcinogenesis, but the precise mechanisms by which it does so are unknown. It was found that TLR9 expression levels were upregulated with increasing severity of colorectal lesions and that TLR9 interacted with NF-κB, suggesting that TLR9 expression might be consistently activated during colitis-CRC progression and that it may promote CAC through NF-κB signaling.125 Furthermore, CpG oligodeoxynucleotide (CpG ODN) stimulation of cell surface TLR9 promotes CRC cell proliferation, and these CpG-ODN TLR9 agonists reduce the cytotoxicity of the anticancer drug adriamycin.126 Another study discovered that TLR-9 mRNA expression was lower in hyperplastic and adenomatous patient cases compared with normal subjects, implying that lower TLR9 expression may promote the progression of polyps into CRC and malignancy.127 Furthermore, as previously stated, TLR9 agonist therapy is now in clinical trials. More research into the role and specific mechanism of TLR9 in CRC is required.
Conclusion
Colorectal cancer is one of the most common human tumors, but its treatment and outcome are unsatisfactory. The presence of a large number of microbiota in the intestine is involved in establishing intestinal homeostasis and disease development, which is linked to the pathogenesis of colorectal cancer, and microbiota dysbiosis may trigger the malignant transformation of colon cells. TLR plays an essential role in developing inflammatory diseases and CRC by regulating intestinal barrier permeability and maintaining intestinal microbial homeostasis. In contrast, as a source of TLR ligands, the intestinal microbiota plays an essential role in TLR ligand activation and distribution. A better understanding of the relationship between gut microbiota, TLR, and CRC will lead to new directions in CRC prevention and treatment.
Acknowledgments
We thank our colleagues for their assistance and constant support.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Training Project of Key Talents of Youth Medicine in Jiangsu province, China (No. QNRC2016330) and Graduate Research- Innovation Project in Jiangsu province (SJCX22_1816), the Key Disease Standardization Diagnosis and Treatment Project in Jiangsu province (N0.BE2015664), the Academic Science and Technology Innovation Fund for College Students (No. x20180714) the Social Development-Health Care Project of Yangzhou, Jiangsu Province (No. YZ2018087), the Social Development Project of Yangzhou, Jiangsu Province (No. YZ2021075), the Graduate Research and Practice Innovation Plan of Graduate Education Innovation Project in Jiangsu Province (N0. SJCX211644), and High-level talent “six one projects” top talent scientific research project of Jiangsu Province (No. LGY2019034), Social development project of key R & D plan of Jiangsu Provincial Department of science and technology (BE2022773). The funding bodies had no role in writing the manuscript.
Author Contributions: Yongkun Fang, Cheng Yan, Qi Zhao contributed equally to this article and participated in writing it. Bin Zhao, Yiqun Liao, Yuji Chen, participated in drawing the pictures in this article and gave guidance, Daorong Wang, Dong Tang gave constructive comments on the structure of the whole article.
All authors read and approved the final .
ORCID iDs: Yongkun Fang  https://orcid.org/0000-0002-6999-4459
https://orcid.org/0000-0002-6999-4459
Dong Tang  https://orcid.org/0000-0002-2057-2968
https://orcid.org/0000-0002-2057-2968
References
- 1. Keum N, Giovannucci E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179-2185. [DOI] [PubMed] [Google Scholar]
- 3. Huang P, Liu Y. A reasonable diet promotes balance of intestinal microbiota: Prevention of precolorectal cancer. Biomed Res Int. 2019;2019:3405278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin Gastroenterol Hepatol. 2019;17:275-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abu-Ghazaleh N, Chua WJ, Gopalan V. Intestinal microbiota and its association with colon cancer and red/processed meat consumption. J Gastroenterol Hepatol. 2021;36:75-88. [DOI] [PubMed] [Google Scholar]
- 6. Jalanka-Tuovinen J, Salonen A, Nikkilä J, et al. Intestinal microbiota in healthy adults: Temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS ONE. 2011;6:e23035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lauka L. Role of the intestinal microbiome in colorectal cancer surgery outcomes. World J Surg Oncol. 2019;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The Human Microbiome Project Consortium. Structure function diversity of the healthy human microbiome. Nature. 2012;486:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pushpanathan P, Mathew GS, Selvarajan S, Seshadri KG, Srikanth P. Gut microbiota and its mysteries. Indian J Med Microbiol. 2019;37:268-277. [DOI] [PubMed] [Google Scholar]
- 10. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physioll Rev. 2010;90:859-904. [DOI] [PubMed] [Google Scholar]
- 11. Molska M, Reguła J. Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients. 2019;11:2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saus E, Iraola-Guzmán S, Willis JR, Brunet-Vega A, Gabaldón T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol Aspects Med. 2019;69:93-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The Influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin C, Cai X, Zhang J, et al. Role of gut microbiota in the development and treatment of colorectal cancer. Digestion. 2019;100:72-78. [DOI] [PubMed] [Google Scholar]
- 16. Mizutani S, Yamada T, Yachida S. Significance of the gut microbiome in multistep colorectal carcinogenesis. Cancer Sci. 2020;111:766-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Werling D, Jungi TW. TOLL-like receptors linking innate and adaptive immune response. Vet Immunol Immunopathol. 2003;91:1-12. [DOI] [PubMed] [Google Scholar]
- 18. Sameer AS, Nissar S. Toll-Like Receptors (TLRs): Structure, functions, signaling, and role of their polymorphisms in colorectal cancer susceptibility. Biomed Res Int. 2021;2021:1157023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kordjazy N. Role of Toll-like receptors in inflammatory bowel disease. Pharmacol Res. 2018;129:204-215. [DOI] [PubMed] [Google Scholar]
- 20. Meng C, Bai C, Brown TD, Hood LE, Tian Q. Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinformatics. 2018;16:33-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bose M, Mukherjee P. Role of microbiome in modulating immune responses in cancer. Mediators Inflamm. 2019;2019:4107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. 2017;67:326-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luddy KA, Robertson-Tessi M, Tafreshi NK, Soliman H, Morse DL. The role of Toll-like receptors in colorectal cancer progression: Evidence for epithelial to leucocytic transition. Front Immunol. 2014;5:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637-650. [DOI] [PubMed] [Google Scholar]
- 25. Schaefer L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237-35245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arias Á. Toll-like receptors-mediated pathways activate inflammatory responses in the esophageal mucosa of adult eosinophilic esophagitis. Clin Transl Gastroenterol. 2018;9:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2015;109:141211-141210. [DOI] [PubMed] [Google Scholar]
- 28. Lavelle EC, Murphy C, O’Neill LA, Creagh EM. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2010;3:17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patin EC, Thompson A, Orr SJ. Pattern recognition receptors in fungal immunity. Semin Cell Dev Biol. 2019;89:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pandey S, Kawai T, Akira S. Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harb Perspect Biol. 2014;7:a016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805-820. [DOI] [PubMed] [Google Scholar]
- 32. O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353-364. [DOI] [PubMed] [Google Scholar]
- 33. Mallard C. Innate immune regulation by Toll-like receptors in the brain. ISRN Neurol. 2012;2012:701950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kawagoe T, Sato S, Matsushita K, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684-691. [DOI] [PubMed] [Google Scholar]
- 35. Deguine J, Barton GM. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thada S, Valluri VL, Gaddam SL. Influence of Toll-like receptor gene polymorphisms to tuberculosis susceptibility in humans. Scand J Immunol. 2013;78:221-229. [DOI] [PubMed] [Google Scholar]
- 38. Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4:a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [DOI] [PubMed] [Google Scholar]
- 40. Morrison DK. MAP kinase pathways. Cold Spring Harb Perspect Biol. 2012;4:a011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ippagunta SK. Identification of Toll-like receptor signaling inhibitors based on selective activation of hierarchically acting signaling proteins. Sci Signal. 2018;11:eaaq1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dowling JK, Mansell A. Toll-like receptors: The swiss army knife of immunity and vaccine development. Clin Transl Immunology. 2016;5:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cui J, Chen Y, Wang HY, Wang RF. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum Vaccin Immunother. 2014;10:3270-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anwar MA, Shah M, Kim J, Choi S. Recent clinical trends in Toll-like receptor targeting therapeutics. Med Res Rev. 2019;39:1053-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rogier EW. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A. 2014;111:3074-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Relman DA. The human microbiome: Ecosystem resilience and health. Nutr Rev. 2012:S2-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Faith JJ. The long-term stability of the human gut microbiota. Science (New York, NY). 2013;341:1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu Z, Cao AT, Cong Y. Microbiota regulation of inflammatory bowel disease and colorectal cancer. Semin Cancer Biol. 2013;23:543-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Artis D, Grencis RK. The intestinal epithelium: Sensors to effectors in nematode infection. Mucosal Immunol. 2008;1:252-264. [DOI] [PubMed] [Google Scholar]
- 51. Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the intricate interaction among Toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J Immunol Res. 2015;2015:489821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lundin A, Bok CM, Aronsson L, et al. Gut flora, Toll-like receptors and nuclear receptors: A tripartite communication that tunes innate immunity in large intestine. Cell Microbiol. 2008;10:1093-1103. [DOI] [PubMed] [Google Scholar]
- 53. Hörmann N, Brandão I, Jäckel S, et al. Gut microbial colonization orchestrates TLR2 expression, signaling and epithelial proliferation in the small intestinal mucosa. PLoS ONE. 2014;9:e113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heimesaat MM, Nogai A, Bereswill S, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59:1079-1087. [DOI] [PubMed] [Google Scholar]
- 55. Kamdar K. Innate recognition of the microbiota by TLR1 promotes epithelial homeostasis and prevents chronic inflammation. J Immunol (Baltimore, MD). 2018;201:230-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burgueño JF, Abreu MT. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol. 2020;17:263-278. [DOI] [PubMed] [Google Scholar]
- 57. Slack E. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science (New York, NY). 2009;325:617-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell Toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147:1363-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vijay-Kumar M, Sanders CJ, Taylor RT, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vijay-Kumar M. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science (New York, NY). 2010;328:228-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cario E. Toll-like receptors in inflammatory bowel diseases: A decade later. Inflamm Bowel Dis. 2010;16:1583-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pimentel-Nunes P, Gonçalves N, Boal-Carvalho I, et al. Decreased Toll-interacting protein and peroxisome proliferator-activated receptor γ are associated with increased expression of Toll-like receptors in colon carcinogenesis. J Clin Pathol. 2012;65:302-308. [DOI] [PubMed] [Google Scholar]
- 63. Lu CC. Upregulation of TLRs and IL-6 as a marker in human colorectal cancer. Int J Mol Sci. 2014;16:159-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dajon M, Iribarren K, Cremer I. Toll-like receptor stimulation in cancer: A pro- and anti-tumor double-edged sword. Immunobiology. 2017;222:89-100. [DOI] [PubMed] [Google Scholar]
- 65. So EY, Ouchi T. The application of Toll like receptors for cancer therapy. Int J Biol Sci. 2010;6675-6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Means AL, Freeman TJ, Zhu J, et al. Epithelial smad4 deletion up-regulates inflammation and promotes inflammation-associated cancer. Cell Mol Gastroenterol Hepatol. 2018;6:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lichtenstern CR, Ngu RK, Shalapour S, Karin M. Immunotherapy, inflammation and colorectal cancer. Cells. 2020;9:618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hnatyszyn A, Hryhorowicz S, Kaczmarek-RyÅ› M, et al. Colorectal carcinoma in the course of inflammatory bowel diseases. Hered Cancer Clin Pract. 2019;17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Friis S, Riis AH, Erichsen R, Baron JA, Sørensen HT. Low-dose aspirin or nonsteroidal anti-inflammatory drug use and colorectal cancer risk: A population-based, case-control study. Ann Intern Med. 2015;163:347-355. [DOI] [PubMed] [Google Scholar]
- 71. Lu Y, Li X, Liu S, Zhang Y, Zhang D. Toll-like receptors and inflammatory bowel disease. Front Immunol. 2018;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine. 2010;491-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wagner H. Endogenous TLR ligands and autoimmunity. Adv Immunol. 2006;91:159-173. [DOI] [PubMed] [Google Scholar]
- 74. Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Molecular Cancer. 2013;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cho M, Carter J, Harari S, Pei Z. The interrelationships of the gut microbiome and inflammation in colorectal carcinogenesis. Clin Lab Med. 2014;34:699-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ou C, Zhang H, Sun Z, Li G, Li X, Li X. Toll-like receptors and non-resolving inflammation-related cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40:202-207. [DOI] [PubMed] [Google Scholar]
- 77. Yeh DW, Huang LR, Chen YW, Huang CF, Chuang TH. Interplay between inflammation and stemness in cancer cells: The role of Toll-like receptor signaling. J Immunol Res. 2016;2016:4368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646-674. [DOI] [PubMed] [Google Scholar]
- 79. O’Leary DP, Bhatt L, Woolley JF, et al. TLR-4 signalling accelerates colon cancer cell adhesion via NF-κB mediated transcriptional up-regulation of Nox-1. PLoS ONE. 2012;7:e44176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dutta J, Fan Y, Gupta N, Fan G, Gélinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800-6816. [DOI] [PubMed] [Google Scholar]
- 81. Fukata M, Chen A, Klepper A, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang S. Tumor evasion of the immune system: Inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood. 2006;107:2432-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang L. Toll-like receptor-4 signaling in mantle cell lymphoma: Effects on tumor growth and immune evasion. Cancer. 2013;119:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fukata M, Chen A, Vamadevan AS, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huang B. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Research. 2005;65:5009-5014. [DOI] [PubMed] [Google Scholar]
- 86. Stier S, Maletzki C, Klier U, Linnebacher M. Combinations of TLR ligands: A promising approach in cancer immunotherapy. Clin Dev Immunol. 2013;2013:271246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kaczanowska S, Joseph AM, Davila E. TLR agonists: Our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93:847-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shi M, Chen X, Ye K, Yao Y, Li Y. Application potential of Toll-like receptors in cancer immunotherapy: Systematic review. Medicine (Baltimore). 2016;95:e3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Matijevic T, Pavelic J. Toll-like receptors: Cost or benefit for cancer? Curr Pharm Des. 2010;16:1081-1090. [DOI] [PubMed] [Google Scholar]
- 90. Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science (New York, NY). 2010;327:291-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Eiró N, González L, González LO, et al. Study of the expression of Toll-like receptors in different histological types of colorectal polyps and their relationship with colorectal cancer. J Clin Immunol. 2012;32:848-854. [DOI] [PubMed] [Google Scholar]
- 92. Weihrauch MR. Phase I clinical study of the Toll-like receptor 9 agonist MGN1703 in patients with metastatic solid tumours. Eur J Cancer (Oxford, England). 2015;51:146-156. [DOI] [PubMed] [Google Scholar]
- 93. Schmoll HJ, Wittig B, Arnold D, et al. Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: A randomised, double-blind, placebo-controlled trial. J Cancer Res Clin Oncol. 2014;140:1615-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Karapetyan L, Luke JJ, Davar D. Toll-like receptor 9 agonists in cancer. Onco Targets Ther. 2020;13:10039-10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cen X, Liu S, Cheng K. The Role of Toll-like receptor in inflammation and tumor immunity. Front Pharmacol. 2018;9:878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang H. TLR2 plays a pivotal role in mediating mucosal serotonin production in the gut. J Immunol (Baltimore, MD). 2019;202:3041-3052. [DOI] [PubMed] [Google Scholar]
- 97. Jia YP, Wang K, Zhang ZJ, et al. TLR2/TLR4 activation induces tregs and suppresses intestinal inflammation caused by Fusobacterium nucleatum in vivo. PLoS ONE. 2017;12:e0186179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Oliveira-Nascimento L, Massari P, Wetzler LM. The Role of TLR2 in infection and immunity. Front Immunol. 2012;3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li Q, Hu L, Yang P, et al. Expression of TLR2/4 in the sperm-storing oviduct of the Chinese soft-shelled turtle Pelodiscus sinensis during hibernation season. Ecol Evol. 2015;5:4466-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Niedzielska I. Toll-like receptors and the tendency of normal mucous membrane to transform to polyp or colorectal cancer. J Physiol Pharmacol. 2009;60:65-71. [PubMed] [Google Scholar]
- 101. Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24-32. [DOI] [PubMed] [Google Scholar]
- 102. Basith S, Manavalan B, Yoo TH, Kim SG, Choi S. Roles of Toll-like receptors in cancer: A double-edged sword for defense and offense. Arch Pharm Res. 2012;35:1297-1316. [DOI] [PubMed] [Google Scholar]
- 103. Botos I, Segal DM, Davies DR. The structural biology of Toll-like receptors. Structure (London, England). 2011;19:447-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chang ZL. Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm Res. 2010;59:791-808. [DOI] [PubMed] [Google Scholar]
- 105. Ding R, Jiao A, Zhang B. Targeting Toll-like receptors on T cells as a therapeutic strategy against tumors. Int Immunopharmacol. 2022;107:108708. [DOI] [PubMed] [Google Scholar]
- 106. Kumar V, Barrett JE. Toll-Like Receptors (TLRs) in health and disease: An overview. Handb Exp Pharmacol. 2022;276:1-21. [DOI] [PubMed] [Google Scholar]
- 107. Medvedev AE. Toll-like receptor polymorphisms, inflammatory and infectious diseases, allergies, and cancer. J Interferon Cytokine Res. 2013;33:467-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ohto U. Conservation and divergence of ligand recognition and signal transduction mechanisms in Toll-like receptors. Chem Pharm Bull (Tokyo). 2017;65:697-705. [DOI] [PubMed] [Google Scholar]
- 109. Sandor F, Buc M. Toll-like receptors. I. Structure, function and their ligands. Folia biologica. 2005;51:148-157. [PubMed] [Google Scholar]
- 110. Meng S, Li Y, Zang X, Jiang Z, Ning H, Li J. Effect of TLR2 on the proliferation of inflammation-related colorectal cancer and sporadic colorectal cancer. Cancer Cell Int. 2020;20:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lowe EL. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS ONE. 2010;5:e13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Liu YD, Ji CB, Li SB, et al. Toll-like receptor 2 stimulation promotes colorectal cancer cell growth via PI3K/Akt and NF-κB signaling pathways. Int Immunopharmacol. 2018;59:375-383. [DOI] [PubMed] [Google Scholar]
- 113. Wang Y. Regional mucosa-associated microbiota determine physiological expression of TLR2 and TLR4 in murine colon. PLoS ONE. 2010;5:e13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tang XY, Zhu YQ, Wei B, Wang H. Expression and functional research of TLR4 in human colon carcinoma. Am J Med Sci. 2010;339:319-326. [DOI] [PubMed] [Google Scholar]
- 115. Gunter MJ. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Research. 2006;66:2483-2487. [DOI] [PubMed] [Google Scholar]
- 116. Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science (New York, NY). 2007;317:124-127. [DOI] [PubMed] [Google Scholar]
- 117. Wang EL. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br J Cancer. 2010;102:908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nihon-Yanagi Y, Terai K, Murano T, Matsumoto T, Okazumi S. Tissue expression of Toll-like receptors 2 and 4 in sporadic human colorectal cancer. Cancer Immunol Immunother. 2012;61:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010-7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yu M, Levine SJ. Toll-like receptor, RIG-I-like receptors and the NLRP3 inflammasome: Key modulators of innate immune responses to double-stranded RNA viruses. Cytokine Growth Factor Rev. 2011;22:63-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Slattery ML, Herrick JS, Bondurant KL, Wolff RK. Toll-like receptor genes and their association with colon and rectal cancer development and prognosis. Int J Cancer. 2012;130:2974-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: TLR3 agonists in cancer therapy. Oncoimmunology. 2020;9:1771143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Connolly DJ, O’Neill LA. New developments in Toll-like receptor targeted therapeutics. Curr Opin Pharmacol. 2012;12:510-518. [DOI] [PubMed] [Google Scholar]
- 124. Suthers AN, Sarantopoulos S. TLR7/TLR9- and B cell receptor-signaling crosstalk: Promotion of potentially dangerous B cells. Front Immunol. 2017;8:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Luo Q, Zeng L, Tang C, Zhang Z, Chen Y, Zeng C. TLR9 induces colitis-associated colorectal carcinogenesis by regulating NF-κB expression levels. Oncol Lett. 2020;20:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nojiri K, Sugimoto K, Shiraki K, et al. The expression and function of Toll-like receptors 3 and 9 in human colon carcinoma. Oncol Rep. 2013;29:1737-1743. [DOI] [PubMed] [Google Scholar]
- 127. Rezasoltani S. Investigating the TLR9 mRNA expression level in different histological types of colorectal polyps. Asian Pac J Cancer Prev. 2019;20:2299-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]