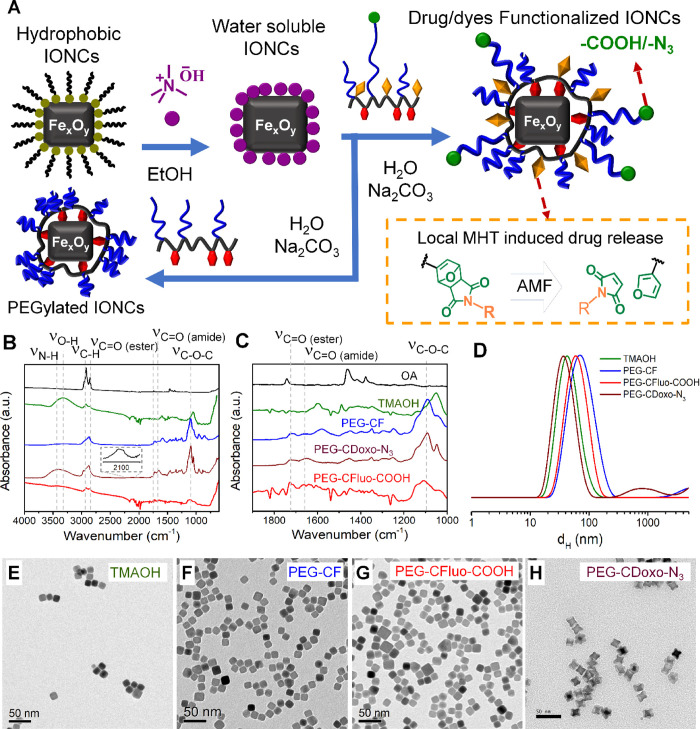
Figure 2.
Phase transfer of iron oxide nanocubes (IONCs) using a two-step ligand exchange. (A) Sketch represents the two-step phase transfer procedure involving first the transfer of IONCs from chloroform into water using tetramethylammonium hydroxide (TMAOH), followed by the postexchange in water of TMAOH with the developed ligands in basic solution, to yield physiologically stable IONCs. The dye/drug conjugated to the ligand platform via a thermal labile Diels–Alder adduct could be released by the local heat generated on the nanocube surface during MHT, as illustrated in the inset. FT-IR spectra of surface modification of IONCs for each step of the water transfer protocol (B) and in the extended region of interest from 1000 to 1900 cm–1 (C). Dynamic light scattering (DLS) traces of water-soluble IONCs modified with TMAOH (green), PEG-CF (blue), PEG-CFluo-COOH (red), and PEG-CDoxo-N3 (deep red) weighted by intensity (D). TEM images of IONCs functionalized with TMAOH (E), or with PEG-CF (F), or with PEG-CFluo-COOH (G), or with PEG-CDoxo-N3 (H) deposited from water.