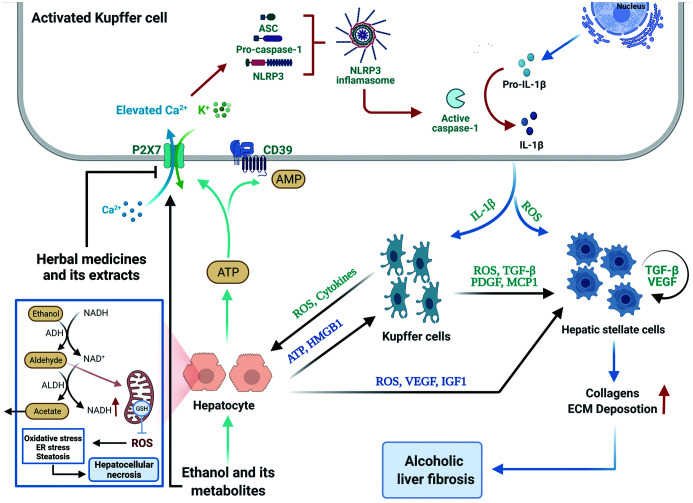
Fig. 2. Schematic diagram of the P2X7 receptor/NLRP3 inflammasome axis involved in the pathogenesis of ALD and evolution into ASH and alcoholic liver fibrosis.
Ethanol, oxidative metabolites of ethanol and lipid metabolism disorders can cause excessive production of mtROS, resulting in oxidative stress, ER stress, and steatosis of liver cells; acetaldehyde depletes the glutathione in the mitochondria, making hepatocytes more sensitive to oxidative stress, and ultimately leads to hepatocellular necrosis. The necrotic hepatocytes can release ATP, which activates P2X7R on KCs, thus opening cationic channels and mediating K+ efflux and Ca2+ influx. K+ efflux and Ca2+ influx in the intracellular environment activate the NLRP3 inflammasome assembly, further activating caspase-1 and mediating IL-1β production and release. Il-1β secreted by activated KCs activates resting KCs, further amplifying the alcohol-induced inflammatory response. The ROS and cytokines secreted by it can further aggravate the damage to hepatocytes, and the secreted ROS, TGF-β and PDGF can promote the activation and proliferation of HSCs. In addition, necrotic hepatocytes release HMPB1, which promotes the inflammatory response of macrophages. Necrotic hepatocytes also secrete ROS, VEGF and IGF1 to promote the active proliferation of HSCs. Activated HSCs (myofibroblasts) secrete excessive collagen and ECM, leading to alcoholic liver fibrosis. Created with BioRender.com