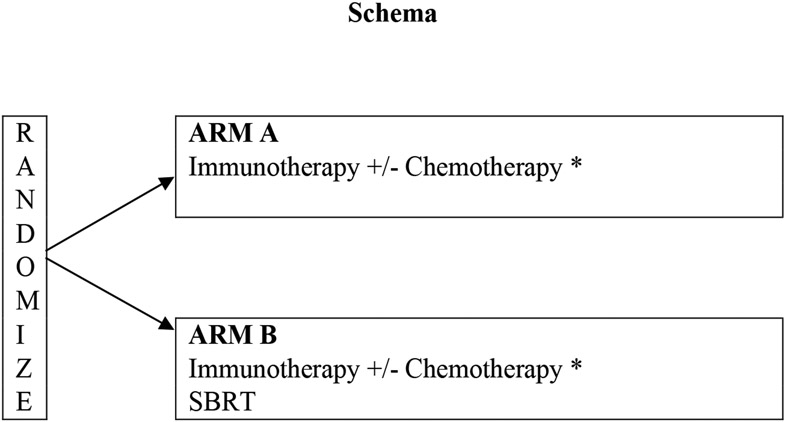
Figure 1: Schema and Systemic Option for ALLIANCE A082002 Trial:
Treatment is to continue for up to 24 months or until disease progression or unacceptable adverse event. Patients will be followed for 5 years or until death, whichever comes first.
*Nivolumab/Ipilimumab (for squamous or non-squamous)
*Pembrolizumab/Carboplatin/Pemetrexed (for non-squamous)
*Nivolumab/Ipilimumab/Carboplatin/Pemetrexed (for non-squamous)
*Pembrolizumab/Carboplatin /Paclitaxel (for squamous)
*Pembrolizumab/Carboplatin/Nab-paclitaxel (for squamous)
*Nivolumab/Ipilimumab/Carboplatin/Paclitaxel (for squamous)