Visual Abstract
Keywords: PSMA, prostate-specific membrane antigen, PET, multiparametric MRI, prostate cancer, diagnosis
Abstract
Multiparametric MRI (mpMRI) is validated for the diagnosis of clinically significant prostate cancer (csPCa). 68Ga-PSMA-11 PET/CT (68Ga-PSMA PET/CT) combined with mpMRI has improved negative predictive value over mpMRI alone for csPCa. The aim of this post hoc analysis of the PRIMARY study was to evaluate the clinical significance of patterns of intraprostatic PSMA activity, proposing a 5-point PRIMARY score to optimize the accuracy of 68Ga-PSMA PET/CT for csPCa in a low-prevalence population. Methods: The PRIMARY trial was a prospective multicenter phase II imaging trial that enrolled men with suspected PCa, no prior biopsy, and a recent mpMRI examination (6 mo) and for whom prostate biopsy was planned. In total, 291 men underwent mpMRI, 68Ga-PSMA PET/CT, and systematic biopsy with or without targeted biopsy. The mpMRI was read separately using the Prostate Imaging Reporting and Data System (PI-RADS) (version 2). 68Ga-PSMA PET/CT (pelvis only) was acquired a minimum of 60 min after injection. 68Ga-PSMA PET/CT was centrally read for pattern (diffuse transition zone [TZ], symmetric central zone [CZ], focal TZ, or focal peripheral zone [PZ]) and intensity (SUVmax). In this post hoc analysis, a 5-level PRIMARY score was assigned on the basis of analysis of the central read: no pattern (score of 1), diffuse TZ or CZ (not focal) (score of 2), focal TZ (score of 3), focal PZ (score of 4), or an SUVmax of at least 12 (score of 5). Two further readers independently assigned a PRIMARY score to 118 scans to determine interrater agreement. Associations between PRIMARY score and csPCa (International Society of Urological Pathology grade group ≥ 2) were evaluated. Results: Of the 291 men enrolled, 162 (56%) had csPCa. A PRIMARY score of 1 was present in 16% (47); a score of 2, in 19% (55); a score of 3, in 10% (29); a score of 4 in 40% (117); and a score of 5, in 15% (43). The proportion of patients with csPCa and a PRIMARY score of 1, 2, 3, 4, and 5 was 8.5% (4/47), 27% (15/55), 38% (11/29), 76% (89/117), and 100% (43/43), respectively. Sensitivity, specificity, positive predictive value, and negative predictive value for a PRIMARY score of 1 or 2 (low-risk patterns) versus a PRIMARY score of 3–5 (high-risk patterns) were 88%, 64%, 76%, and 81%, respectively, compared with 83%, 53%, 69%, and 72%, respectively, for a PI-RADS score of 2 versus 3–5 on mpMRI. The Cohen κ for a PRIMARY score of 1 of 2 versus a PRIMARY score of 3–5 was 0.76 (95% CI, 0.64–0.88) for reader 1 and 0.64 (95% CI, 0.49–0.78) for reader 2. Conclusion: A PRIMARY score incorporating intraprostatic pattern and intensity on 68Ga-PSMA PET/CT shows potential, with high diagnostic accuracy for csPCa. Further validation is warranted before implementation.
Multiparametric MRI (mpMRI) is currently the standard of care for the diagnosis of prostate cancer, with validated standardization of reporting using the Prostate Imaging Reporting and Data System (PI-RADS), version 2 (1). However, 68Ga-PSMA-11 PET/CT (68Ga-PSMA PET/CT) has recently been reported to demonstrate similar diagnostic accuracy to MRI for the diagnosis of prostate cancer, with significant improvement in negative predictive value if the 2 modalities are used in combination (2). The specificity of 68Ga-PSMA PET/CT for clinically significant prostate cancer (csPCa) in the PRIMARY trial was lower than previously published, as is likely due to the lower prevalence of csPCa (3,4). In a screening setting, PSMA activity in intraprostatic processes such as benign prostatic hypertrophy, prostate intraepithelial neoplasia, and low-grade International Society of Urological Pathology (ISUP) grade group 1 malignancy can be difficult to distinguish from csPCa on the basis of PSMA intensity alone. Detection of tumor on intraprostatic 68Ga-PSMA PET/CT currently depends on a higher level of uptake in the tumor than in the background. By contrast, the PI-RADS system utilizes intraprostatic anatomy, differentiating the peripheral zone (PZ) and transition zone (TZ) to better categorize the likelihood of malignancy. Because most prostate cancers arise within the PZ, incorporating anatomic differentiation helps improve diagnostic certainty. It is unknown whether the incorporation of anatomic localization and pattern characterization can improve the diagnostic accuracy of 68Ga-PSMA PET/CT in a prebiopsy patient population. The aim of this post hoc analysis of the PRIMARY study was to explore the value of intraprostatic 68Ga-PSMA PET/CT patterns and intensity scores, developing a 5-level score to improve diagnostic accuracy for csPCa.
MATERIALS AND METHODS
Study Design
The PRIMARY trial was a prospective multicenter, phase II imaging trial conducted across 3 academic institutions in Australia (5). The study protocol was approved by the St. Vincent’s Hospital institutional review board (HREC/18/SVH/239), and all patients provided written informed consent. The study was registered with ANZCTR (ANZCTRN12618001640291), and the protocol and initial results were previously published (2,5). This was a post hoc analysis of the PRIMARY trial to evaluate the diagnostic performance of a 5-point scoring system to detect csPCa.
Screening
Men were considered eligible for the trial if there was clinical suspicion of prostate cancer based on an abnormal prostate-specific antigen level (<20 ng/mL) or abnormal results on digital rectal examination after assessment by a study urologist. Men were excluded if they had a prior diagnosis of prostate cancer, a prior prostate biopsy, or prior prostate MRI. All men underwent mpMRI within 6 mo and consented to transperineal systematic biopsy with or without targeted biopsy. Men who had low-risk MRI findings (PI-RADS score of 1) were not enrolled, nor were men who had a PI-RADs score of 2 with low-risk clinical features and no planned biopsy.
MRI
mpMRI was performed and reported locally by the prostate-MRI subspecialist radiologist preferred by the urology investigator, with the findings reported per PI-RADS version 2. Whenever a lesion was identified on mpMRI, images were provided to the treating investigator for MRI-targeted biopsy. The PI-RADS score and location were documented for each lesion, in addition to overall prostate volume. A PI-RADS score of 3–5 was defined as positive for analysis.
68Ga-PSMA PET Acquisition
Pelvis-only 68Ga-PSMA PET/CT was performed at a minimum of 60 min after administration of a 1.8–2.2 MBq/kg dose of 68Ga-PSMA, using a low-dose unenhanced-CT protocol (3 min per bed position). This limited protocol reduced the radiation dose to less than 4 mSv, appropriate to the screening context. PET/CT cameras at all 3 participating centers were harmonized via standardized phantom calibration through the Australasian Radiopharmaceutical Trials Network. An initial local PSMA read was done, and local readers provided key images to treating urologists to allow PSMA targeting at biopsy. If pelvic lymph nodes and metastatic disease were identified and documented, the sites performed whole-body 68Ga-PSMA PET outside the protocol (6.2% of patients had additional whole-body 68Ga-PSMA PET).
68Ga-PSMA PET Interpretation
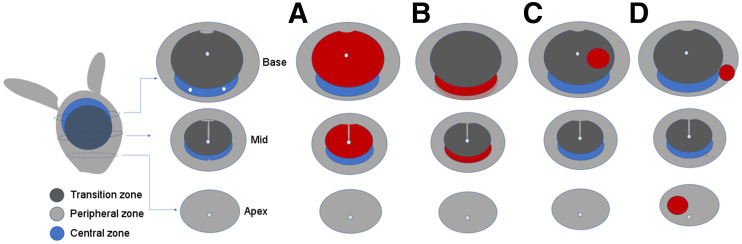
All 68Ga-PSMA PET scans were centrally read by 2 experienced nuclear medicine specialists masked to the previous MRI and clinical outcomes. The PZ, central zone (CZ), and TZ were differentiated using the PET/CT images and known anatomic definitions of zonal boundaries (Fig. 1). Readers interrogated the 68Ga-PSMA PET/CT scans for specific patterns: diffuse TZ activity (pattern A), symmetric CZ activity (pattern B), focal PZ activity (pattern C), or focal TZ activity (pattern D) (Fig. 1). All patterns were documented, as was uptake (SUVmax) in each prostate quadrant, with the single highest value used for analysis.
FIGURE 1.
Anatomic representation of CZ, TZ, and PZ and patterns of intraprostatic PSMA activity: diffuse TZ (A), symmetric CZ (B), focal TZ (C), and focal PZ (D). Simplification of prostate zones was utilized for study, with PZ encompassing prostate PZ margin as defined on 68Ga-PSMA PET/CT, as well as entire apex.
A region was defined as TZ if centrally placed within the prostate, with no PSMA activity extending to the edge of the prostate margin on the CT portion of the fused PET/CT images (Fig. 1, pattern A). Symmetric CZ activity was localized to the CZ, with no PSMA activity extending to the prostate margin on the fused PET/CT images (Fig. 1, pattern B). If symmetric CZ activity extended to the posterior margin of the prostate on the fused PET/CT images, this activity was classified as PZ as well as CZ. Focal activity within the TZ was defined visually as more than twice the background TZ activity (Fig. 1, pattern C). All findings within the prostatic apex were defined as PZ, as were all findings that included the PZ margin of the prostate on the fused PET/CT images (Fig. 1, pattern D). Any focal activity in the PZ was considered abnormal. No SUV minimal threshold was used excepting those lesions that had very high intensity (SUVmax > 12).
A PRIMARY score using a combination of pattern information and SUVmax was assigned to each patient. Score 1 was no pattern and low-grade activity. Score 2 was diffuse TZ or symmetric CZ activity without focal uptake (this included diffuse TZ activity with irregular focal uptake that was not well above background TZ activity). Score 3 was focal TZ activity (focal TZ activity had to be visually greater than twice the background TZ activity). Score 4 was focal PZ activity. Score 5 was any pattern with an SUVmax of at least 12 (Table 1).
TABLE 1.
Patient Characteristics
| Variable | Data |
|---|---|
| Age at biopsy (y) | 64 (59–70) |
| Latest prostate-specific antigen result* (ng/mL) | 5.6 (4.2–7.5) |
| Clinical T-stage | |
| TX | 18 (6.2) |
| T1c | 197 (68) |
| T2a | 60 (21) |
| T2b | 14 (4.8) |
| T2c | 2 (0.7) |
| PI-RADS (mpMRI) | |
| 2 | 95 (33) |
| 3 | 53 (18) |
| 4 | 90 (31) |
| 5 | 53 (18) |
| Grade group (biopsy) | |
| No cancer | 77 (26) |
| 1 | 52 (18) |
| 2 | 102 (35) |
| 3 | 39 (13) |
| 4 | 7 (2.4) |
| 5 | 14 (4.8) |
| PSMA pattern† | |
| No pattern | 47 (16) |
| Diffuse TZ/CZ | 97 (33) |
| Focal TZ | 53 (18) |
| Focal PZ | 155 (53) |
Prostate-specific antigen data missing for one patient.
†Number exceeds sample size because patient may exhibit more than one pattern simultaneously.
Qualitative data are number and percentage; continuous data are median and interquartile range.
In some cases, multiple patterns were identified, and the PRIMARY score represented the most clinically significant pattern (focal pattern above diffuse or symmetric, PZ above TZ, and SUVmax > 12 above any reported pattern). Differentiating CZ activity from PZ activity in the posterior basal prostate (where the PZ may be thin) can be difficult, and if there was any doubt the readers were asked to classify this as a PRIMARY score of 4 rather than 2.
A further 2 masked independent reads were done for a random sample of 120 68Ga-PSMA PET/CT scans by 2 experienced nuclear medicine specialists after a training-set explanation of the PRIMARY 5-point score definitions and criteria. These additional reads were used to determine the interrater variability of the PRIMARY score. No a priori hypothesis for a minimum acceptable concordance measure was considered. Two patients’ images were not immediately available for remote interrogation, leaving 118 pairs of evaluable concordance reads.
Prostate Biopsy and Histopathology
Systematic transperineal prostate biopsies with a recommended minimum 18 cores (dependent on prostate volume) were mandated. Additional targeted biopsies were obtained, when possible, with all urology investigators provided with key images to demonstrate sites of both MRI and 68Ga-PSMA PET abnormalities before biopsy. All biopsies were processed and reported according to grade group protocols by subspecialist uropathologists at each study center. For analysis, any overall ISUP grade group of at least 2 on biopsy (systematic or targeted) was considered csPCa.
Statistical Analysis
In addition to basic descriptive statistics, the areas under the curve for the 5-level PRIMARY score and PI-RADS were compared using the DeLong test, though there was no a priori hypothesis. Interrater agreement was evaluated with the Cohen κ, and associated 95% CIs were provided by the kappaetc command in Stata, version 16.0MP (StataCorp). The interaction between SUVmax and PSMA pattern type (none, nonfocal, focal) was explored in a logistic regression model in which both variables and an interaction term were simultaneously entered. The estimated marginal probabilities of csPCa versus SUVmax were plotted per pattern type. Stata was used for analysis.
RESULTS
In total, 291 men with a median age of 64 y (interquartile range, 59–70 y) underwent MRI, 68Ga-PSMA PET/CT, and biopsy. Of these, 162 (56%) men had clinically significant malignancy (ISUP grade group ≥ 2) on biopsy and 196 (67%) had a positive MRI result (PI-RADS 3–5). Forty-seven patients (16%) had no pattern identified on 68Ga-PSMA PET/CT, 97 (33%) exhibited either diffuse TZ or symmetric CZ activity, 53 (18%) had focal TZ activity, and 155 (53%) had focal activity in the PZ (Table 1).
PRIMARY Score
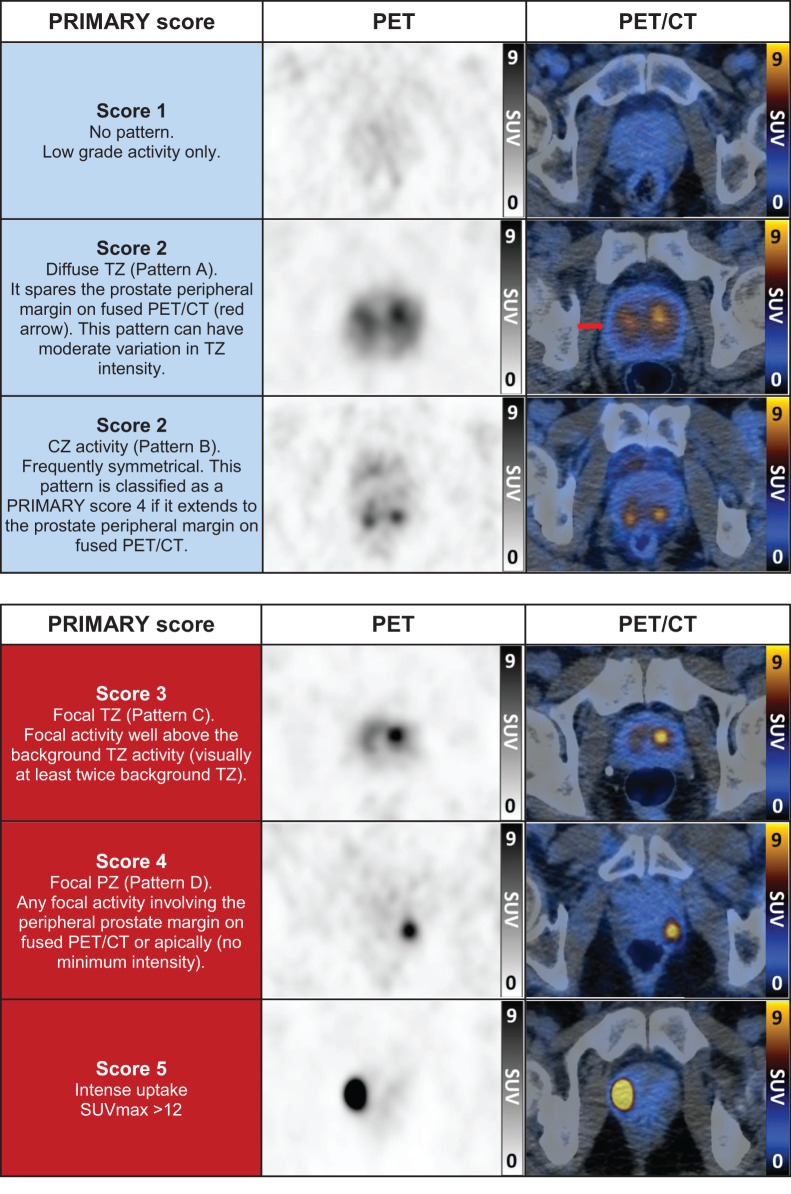
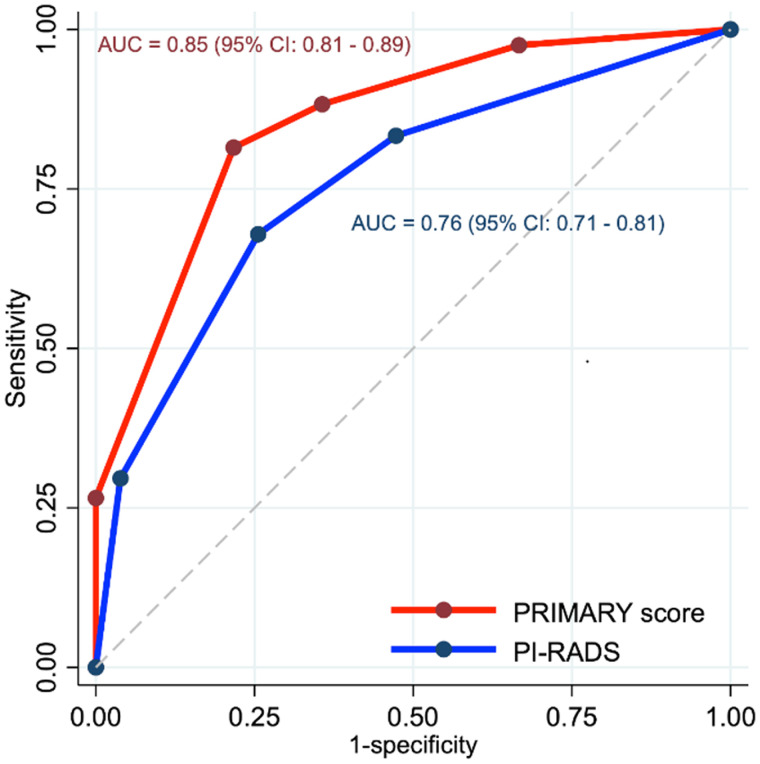
The PRIMARY score distribution was as follows: score 1, 16% (n = 47); score 2, 19% (n = 55); score 3, 10% (n = 29); score 4, 40% (n = 117); and score 5, 15% (n = 43) (Table 2; Fig. 2). The proportion of men with csPCa and a PRIMARY score of 1, 2, 3, 4, or 5 was 8.5% (4/47), 27% (15/55), 38% (11/29), 76% (89/117), and 100% (43/43), respectively. The estimated area under the curve of the 5-level PRIMARY score was 0.85 (95% CI, 0.81–0.89) and exceeded that of PI-RADS, which was 0.76 (95% CI, 0.71–0.81) (P = 0.003) (Fig. 3). Sensitivity, specificity, positive predictive value, and negative predictive value were 88%, 64%, 76%, and 81%, respectively, for a PRIMARY score of 3–5 (high-risk patterns) versus 83%, 53%, 69%, and 72%, respectively, for a PRIMARY score of 1 or 2 (low-risk patterns), for PI-RADS 3–5 versus 2 (Table 3; Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org).
TABLE 2.
PRIMARY Scores
| Score | Description | n | csPCa (%) |
|---|---|---|---|
| 1 | No dominant intraprostatic pattern; low-grade activity | 47 | 8.5 |
| 2 | Diffuse TZ activity or symmetric CZ activity that does not extend to prostate margin on CT | 55 | 27 |
| 3 | Focal TZ activity visually twice background TZ activity | 29 | 38 |
| 4 | Focal PZ activity (no minimum intensity) | 117 | 76 |
| 5 | PSMA SUVmax > 12 | 43 | 100 |
FIGURE 2.
68Ga-PSMA PET/CT examples of PRIMARY scores.
FIGURE 3.
Receiver-operating-characteristic curves for 5-level PRIMARY score and PI-RADS. AUC = area under curve.
TABLE 3.
Diagnostic Performance for PRIMARY Score (1 or 2 vs. 3–5) and PI-RADS (2 vs. 3–5)
| Parameter | PRIMARY score | PI-RADS | ||
|---|---|---|---|---|
| Central read | Reader 1 | Reader 2 | ||
| All patients (n = 291) | ||||
| Sensitivity | 88 (82–93) | 83 (77–89) | ||
| Specificity | 64 (55–73) | 53 (44–62) | ||
| Positive predictive value | 76 (69–82) | 69 (62–75) | ||
| Negative predictive value | 81 (72–88) | 72 (61–80) | ||
| Multiple readers (n = 118) | ||||
| Sensitivity | 88 (77–95) | 83 (71–92) | 92 (82–97) | 82 (70–90) |
| Specificity | 67 (54–79) | 72 (59–83) | 64 (50–76) | 52 (38–65) |
| Positive predictive value | 74 (62–83) | 76 (64–85) | 72 (61–82) | 64 (52–74) |
| Negative predictive value | 85 (71–94) | 81 (67–90) | 88 (74–96) | 73 (57–85) |
| κ PRIMARY score (1 or 2 vs. 3–5) | 0.76 (0.64–0.88) | 0.64 (0.49–0.78) | ||
Data are percentages, with 95% CIs in parentheses.
Interrater Agreement
Two further readers assessed 68Ga-PSMA PET in 118 patients (51% had csPCa). The Cohen κ for a PRIMARY score of 1 or 2 versus a PRIMARY score of 3–5 was 0.76 (95% CI, 0.64–0.88) for reader 1 and 0.64 (95% CI, 0.49–0.78) for reader 2. The Cohen κ for the 5-point PRIMARY score was 0.73 (95% CI, 0.63–0.83) for reader 1 and 0.56 (95% CI, 0.45–0.68) for reader 2. The diagnostic performance of the PRIMARY score as used by the readers was broadly similar to that of the central read (Table 3).
PRIMARY Score and Overall Grade Group
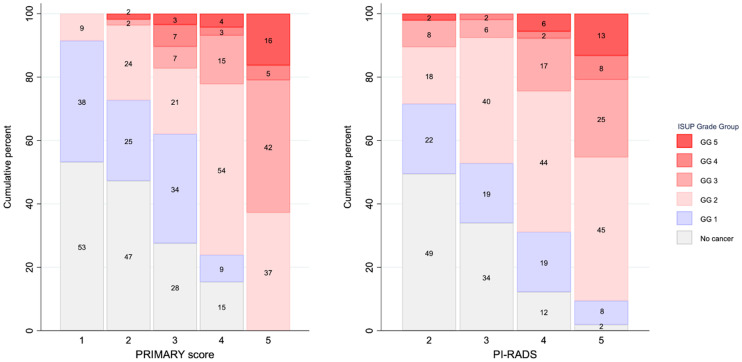
Overall grade group on histopathology was associated with PRIMARY score (Fig. 4). All 4 patients with a PRIMARY score of 1 and csPCa had grade group 2 cancer. For a PRIMARY score of 2, 2 of 55 (4%) had cancer of at least grade group 3. Conversely, for a PRIMARY score of 5, 27 of 43 (63%) had cancer that was at least grade group 3. For PI-RADS 2 patients, 10 of 95 (11%) had cancer that was at least grade group 3, whereas 5 of 53 (9.4%) of PI-RADS 5 patients had no csPCa.
FIGURE 4.
Distribution of overall ISUP grade group by PRIMARY score and PI-RADS. Numbers within bars are percentages. GG = grade group.
Pattern and Intensity
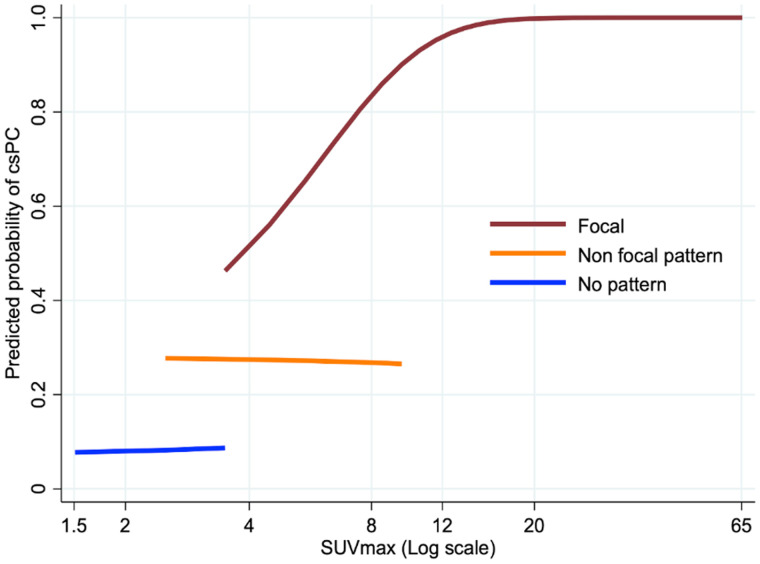
In exploring the effect of SUVmax on prediction of csPCa for different pattern types (none, nonfocal, focal), we found that an increasing SUVmax was associated with a higher likelihood of malignancy only with focal patterns. Increasing SUVmax did not raise the predicted probability of csPCa in patients without a pattern or those with nonfocal patterns (diffuse TZ or symmetric CZ) (Fig. 5).
FIGURE 5.
Predicted probability of csPCa vs. SUVmax by pattern of uptake plotted for range of SUVmax for that pattern.
DISCUSSION
mpMRI is the accepted standard of care for the diagnosis of prostate cancer, with the PRECISION trial demonstrating improved detection of significant malignancy and a safe reduction in the number of biopsies required relative to prostate biopsy alone (6). 68Ga-PSMA PET/CT has high-level evidence for its use in the staging of prostate cancer (7) and in biochemical recurrence after definitive primary therapy (8–10). However, there is little evidence for its value in the diagnosis of primary tumors (11–13). The PRIMARY trial recently showed that limited-field-of-view pelvic 68Ga-PSMA PET/CT combined with mpMRI significantly improved both sensitivity and negative predictive value compared with mpMRI alone in the diagnosis of prostate cancer (2). This PRIMARY substudy has identified key patterns of intraprostatic PSMA activity, determining those more likely to be benign and those more likely to demonstrate malignancy. The initial PRIMARY study analysis utilized a minimum PSMA SUVmax cutoff (4.0) with expert reader analysis, finding a sensitivity of 90% with a specificity of 50% for csPCa. However, this method is not valid across unharmonized PET cameras and with variable PSMA ligands. Using key patterns within the PRIMARY score in this substudy improved specificity without compromising sensitivity for the diagnosis of csPCa. Further, use of pattern and intensity within a 5-level score (PRIMARY) was reproducible between readers, with an increased or equivalent diagnostic accuracy for the detection of csPCa compared with mpMRI.
PI-RADS is a 5-point scale recommended for reporting of prostate MRI findings by a European consensus meeting in 2011 (14). The PI-RADS system reports by prostatic zone, separating the PZ from the TZ and CZ while incorporating semiquantitative measures such as apparent diffusion coefficient maps and diffusion-weighted imaging. The incidence of prostate malignancy varies by zonal location within the prostate, a fact that is utilized by PI-RADS to improve accuracy. Histopathologic analysis from prostatectomy specimens has found that malignancy arises from the PZ in 68% of cases, the TZ in 24%, and the CZ in 8% (15). The anatomy of the prostate is well described and clearly delineated on reporting aids. The PZ extends posterolaterally around the gland, involving most of the apex. The TZ is centrally placed and enlarges with benign prostatic hypertrophy. The CZ surrounds the ejaculatory duct apparatus and makes up most of the central prostatic base. Zonal anatomy is clearly visible on prostate MRI compared with CT. However, use of the fused PSMA PET/CT images allows basic differentiation between TZ and PZ activity. TZ activity does not extend to the margin of the prostate on CT (leaving a photopenic rim), whereas PZ activity extends fully to the prostate edge. Although prostatic zones are less well demarcated on 68Ga-PSMA PET/CT than on mpMRI, this study has shown that this broad definition of patterns within zones is reproducible and improves diagnostic accuracy for csPCa.
PSMA is a transmembrane glycoprotein highly expressed on the surface of prostate cancer cells. However, the receptor is also expressed in benign pathologies such as benign prostatic hypertrophy and prostate intraepithelial neoplasia (16). Further, expression of PSMA in prostate cancer is a spectrum, with higher-grade pathology expressing higher levels of the receptor than low-grade ISUP grade group 1 malignancy (4). Benign intraprostatic processes can have relatively high PSMA expression, with significant overlap between the PSMA intensity expressed in low-volume malignancy and benign disease. However, the pattern of PSMA activity, in addition to PSMA intensity, appeared effective at differentiating benign causes from significant prostate malignancies in this study. The 2 key low-risk patterns reported included diffuse TZ activity and symmetric CZ activity, both occurring centrally in the prostate. Increased CZ PSMA activity has previously been reported as a potential cause of false-positives (17,18). The incidence of malignancy associated with symmetric CZ activity, although low, is higher than if only low-grade PSMA activity is present, probably because the increased PSMA activity, although benign, may mask focal uptake within small cancers directly adjacent. For this reason, if the CZ activity extended to the posterior margin of the PET/CT images, the study was classified as having a PRIMARY score of 4 (PZ). Although this classification may have reduced the specificity of the PRIMARY score, it ensured that sensitivity for csPCa was maintained.
The proposed PRIMARY score uses a combination of pattern (focal vs. diffuse), zonal location, and high SUVmax to optimize reporting accuracy. The score relies more on pattern than intensity, using only a high SUVmax (≥12) as the top score (PRIMARY score of 5) because of its 100% specificity for the presence of significant malignancy. This reduced reliance on PSMA SUVmax makes the PRIMARY score more applicable across a range of PET cameras and PSMA ligands, with pattern unlikely to change significantly for these reasons. As no minimum intensity level is required in the PZ for a PRIMARY score of 4, and a PRIMARY score of 3 is dependent on background TZ counts, a fixed SUVmax scale should not be used by reporters for the PRIMARY score. Further, a PRIMARY score of 5 (SUVmax ≥ 12) will need to be validated across different 68Ga-PSMA PET ligands but was felt to be an important part of the score. The association between a very high PSMA SUVmax and csPCa is strong and may be valuable as both a diagnostic and a prognostic tool (19).
Interrater reproducibility for differentiating PRIMARY score low-risk from high-risk patterns was substantial between independent readers and equivalent to those that prospective trials previously reported for 68Ga-PSMA PET in biochemical recurrence and in evaluation of lymph node involvement in the staging setting (9,20,21). Interrater concordance was higher than previously reported with PI-RADS version 2 for mpMRI (22). Further, diagnostic performance remained high across all readers using the PRIMARY score.
This study had several limitations. The PRIMARY score was developed and evaluated within the same prospective trial. Although the PRIMARY score results had high interrater reproducibility and accuracy in this population, the score must be validated in other prebiopsy datasets, with further evaluation of both intrareader and interreader reproducibility before clinical implementation. Further, whereas the PRIMARY score relies predominately on intraprostatic patterns, a high SUVmax is included because of its high specificity. Further work is required to validate an optimal high-SUVmax cutoff across camera systems and different PSMA ligands. The option of using liver or parotid activity is not possible because of the use of a pelvis-only 68Ga-PSMA PET/CT protocol to reduce radiation dose as appropriate for a screening setting.
The study was undertaken in a population of men who had undergone mpMRI and for whom transperineal prostate biopsy was planned. This means that men with a PI-RADS score of 1 or a PI-RADS score of 2 with a low clinical risk were not included in the population. This MRI triaging reduced the negative predictive value for PI-RADS from that previously reported in low-prevalence populations (23). However, adding 68Ga-PSMA PET to a low-risk-mpMRI population would not be clinically appropriate, and the finding that 68Ga-PSMA PET/CT is independently accurate for prostate cancer diagnosis in an mpMRI-triaged population is important.
Any ISUP grade group 2 malignancy in the prostate was considered significant. A more detailed analysis of whole-mount histopathology with overlaid PSMA may yield more accurate results. However, this study did not have whole-mount histopathology because many men did not proceed to treatment.
CONCLUSION
A 5-level PRIMARY score incorporating intraprostatic pattern and intensity on 68Ga-PSMA PET/CT shows potential for diagnosing csPCa with high accuracy. Further validation of this scoring system in a screening population is warranted before clinical implementation.
DISCLOSURE
The trial was funded through competitive grants from the St. Vincent’s Curran Foundation and the St. Vincent’s Clinic Foundation, a Cancer Institute of NSW translational grant, and a SPHERE NSW SLBVC grant. Louise Emmett reports grants from Movember, the Cancer Institute New South Wales, and St. Vincent’s Curran and Clinic Foundations; nonfinancial support from Astellas and Endocyte/AAA; and personal fees from Janssen, AstraZeneca, Astellas, Telix, Mundipharma, Bayer, and Amgen. Michael Hofman reports philanthropic or government grant support from the Prostate Cancer Foundation (PCF), CANICA Oslo Norway, the Peter MacCallum Foundation, the Medical Research Future Fund, an NHMRC investigator grant, Movember, the U.S. Department of Defense, and the Prostate Cancer Foundation of Australia (PCFA); grant support from AAA/Novartis; and consulting fees for lectures or advisory boards from Astellas, AstraZeneca, Janssen, Merck/MSD, Mundipharma, and Point Biopharma. Thomas Hope reports grants from the Prostate Cancer Foundation, the National Cancer Institute (R01CA235741 and R01CA212148), and Clovis Oncology and personal fees from RayzeBio, Curium, and BlueEarth Diagnostics, outside the submitted work. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We acknowledge the significant work undertaken for this trial by the nuclear medicine and urology teams across St. Vincent’s Hospital, Sydney; Sir Peter MacCallum Cancer Centre, Melbourne; and Royal Brisbane and Women’s Hospital, Brisbane.
KEY POINTS
QUESTION: Can a 5-point PRIMARY score based on patterns of intraprostatic PSMA activity optimize the accuracy of 68Ga-PSMA PET/CT for diagnosis of csPCa?
PERTINENT FINDINGS: The PRIMARY score was equivalent in diagnostic accuracy to mpMRI alone for the detection of csPCa: sensitivity, specificity, positive predictive value, and negative predictive value for PRIMARY low-risk patterns (score of 3–5) were 88%, 64%, 76%, and 81%, respectively, versus 83%, 53%, 69%, and 72%, respectively, for high-risk patterns (score of 1 or 2), for PI-RADS 3–5 vs. 2 on mpMRI.
IMPLICATIONS FOR PATIENT CARE: A 5-level PRIMARY score incorporating intraprostatic patterns and intensity on 68Ga-PSMA PET/CT shows potential as an accurate method for diagnosing csPCa in screening populations and warrants further validation.
REFERENCES
- 1. Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340–351. [DOI] [PubMed] [Google Scholar]
- 2. Emmett L, Buteau J, Papa N, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): a prospective multicentre study. Eur Urol. 2021;80:682–689. [DOI] [PubMed] [Google Scholar]
- 3. Eiber M, Nekolla SG, Maurer T, Weirich G, Wester HJ, Schwaiger M. 68Ga-PSMA PET/MR with multimodality image analysis for primary prostate cancer. Abdom Imaging. 2015;40:1769–1771. [DOI] [PubMed] [Google Scholar]
- 4. Scheltema MJ, Chang JI, Stricker PD, et al. Diagnostic accuracy of 68Ga-prostate-specific membrane antigen (PSMA) positron-emission tomography (PET) and multiparametric (mp)MRI to detect intermediate-grade intra-prostatic prostate cancer using whole-mount pathology: impact of the addition of 68Ga-PSMA PET to mpMRI. BJU Int. 2019;124(suppl 1):42–49. [DOI] [PubMed] [Google Scholar]
- 5. Amin A, Blazevski A, Thompson J, et al. Protocol for the PRIMARY clinical trial, a prospective, multicentre, cross-sectional study of the additive diagnostic value of gallium-68 prostate-specific membrane antigen positron-emission tomography/computed tomography to multiparametric magnetic resonance imaging in the diagnostic setting for men being investigated for prostate cancer. BJU Int. 2020;125:515–524. [DOI] [PubMed] [Google Scholar]
- 6. Kasivisvanathan V, Emberton M, Moore CM. MRI-targeted biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;379:589–590. [DOI] [PubMed] [Google Scholar]
- 7. Hofman MS, Lawrentschuk N, Francis RJ, et al. ; proPSMA Study Group Collaborators. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–1216. [DOI] [PubMed] [Google Scholar]
- 8. Emmett L, Tang R, Nandurkar R, et al. 3-year freedom from progression after 68Ga-PSMA PET/CT-triaged management in men with biochemical recurrence after radical prostatectomy: results of a prospective multicenter trial. J Nucl Med. 2020;61:866–872. [DOI] [PubMed] [Google Scholar]
- 9. Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pienta KJ, Gorin MA, Rowe SP, et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18F-DCFPyL in PROSTATE CANCER PAtients (OSPREY). J Urol. 2021;206:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berger I, Annabattula C, Lewis J, et al. 68Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: correlation with final histopathology. Prostate Cancer Prostatic Dis. 2018;21:204–211. [DOI] [PubMed] [Google Scholar]
- 12. Ferraro DA, Becker AS, Kranzbühler B, et al. Diagnostic performance of 68Ga-PSMA-11 PET/MRI-guided biopsy in patients with suspected prostate cancer: a prospective single-center study. Eur J Nucl Med Mol Imaging. 2021;48:3315–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Yu F, Yang L, et al. 68Ga-PSMA-11 PET/CT combining ADC value of MRI in the diagnosis of naive prostate cancer: perspective of radiologist. Medicine (Baltimore). 2020;99:e20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59:477–494. [DOI] [PubMed] [Google Scholar]
- 15. McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma: correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12:897–906. [DOI] [PubMed] [Google Scholar]
- 16. Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–2261. [DOI] [PubMed] [Google Scholar]
- 17. Ganeshalingam R, Hsiao E. Compressed central zone uptake on PSMA PET/CT: a potential pitfall in interpretation. Clin Nucl Med. 2019;44:570–571. [DOI] [PubMed] [Google Scholar]
- 18. Pizzuto DA, Müller J, Mühlematter U, et al. The central zone has increased 68Ga-PSMA-11 uptake: “Mickey Mouse ears” can be hot on 68Ga-PSMA-11 PET. Eur J Nucl Med Mol Imaging. 2018;45:1335–1343. [DOI] [PubMed] [Google Scholar]
- 19. Roberts MJ, Morton A, Donato P, et al. 68Ga-PSMA PET/CT tumour intensity pre-operatively predicts adverse pathological outcomes and progression-free survival in localised prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:477–482. [DOI] [PubMed] [Google Scholar]
- 20. Ceci F, Oprea-Lager DE, Emmett L, et al. E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging. 2021;48:1626–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hope TA, Eiber M, Armstrong WR, et al. Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: a multicenter prospective phase 3 imaging trial. JAMA Oncol. 2021;7:1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park KJ, Choi SH, Lee JS, Kim JK, Kim MH. Interreader agreement with prostate imaging reporting and data system version 2 for prostate cancer detection: a systematic review and meta-analysis. J Urol. 2020;204:661–670. [DOI] [PubMed] [Google Scholar]
- 23. Sathianathen NJ, Omer A, Harriss E, et al. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the prostate imaging reporting and data system era: a systematic review and meta-analysis. Eur Urol. 2020;78:402–414. [DOI] [PubMed] [Google Scholar]