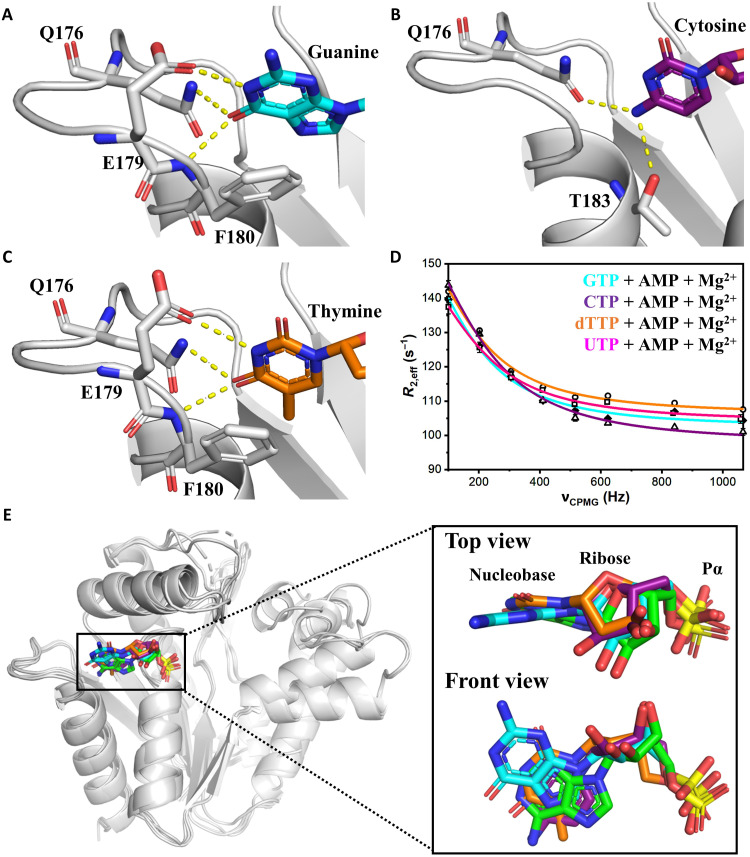
Fig. 5. Structural basis for universal NTP binding by OdinAK.
(A to C) Crystal structures of OdinAK in complex with NTPs demonstrate that the key determinant of selective binding of the nucleobases is the interactions formed by the side chain of Q176. In addition, the interactions formed by other important side chains are indicated in the different complexes. (A) Structure of OdinAK in complex with GTP. (B) Structure of OdinAK in complex with CTP. (C) Structure of OdinAK in complex with dTTP. (D) 19F CPMG RD profiles obtained from fluorinated Y44W for different substrates under turnover conditions (compare to Fig. 4F). The resulting fits from the data (table S2 and Table 2) show that OdinAK is rate-limited by slow conformational dynamics for all NTPs. (E) A key factor for the broad substrate specificity of OdinAK is harnessing of the endogenous flexibility of NTP molecules. Shown is a superimposition of NTP substrates bound to OdinAK. The color coding is as follows: ATP (green), GTP (cyan), CTP (purple), and dTTP (orange). The overlay was generated by superimposing the carbon α atoms of OdinAK, and for simplicity, only the base, ribose, and α phosphate are shown for the NTPs.