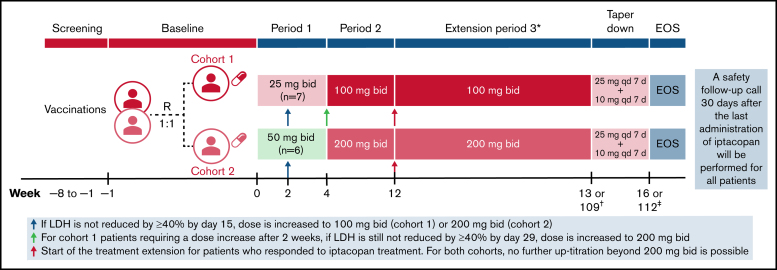
Figure 1.
Study design and iptacopan treatment duration. *Following period 3, patients will also have the possibility to transition directly to a long-term rollover extension program without a need for taper-down. †Week 13 for nonresponders or week 109 for responders. ‡Week 16 for nonresponders or week 112 for responders. bid, twice daily; EOS, end of study; qd, once daily; R, randomization.