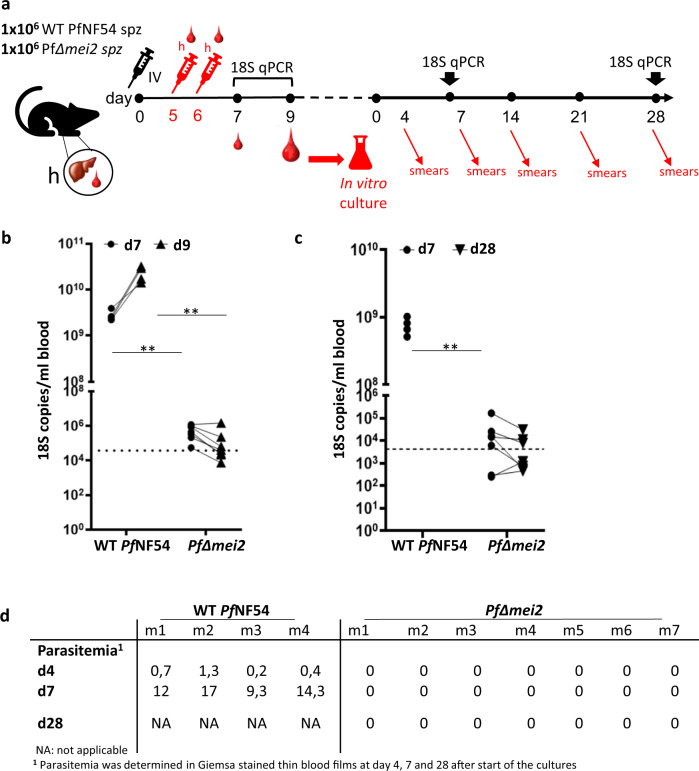
Fig. 3. Development of PfΔmei2 in FRG huHep mice containing human red blood cells.
a Timeline of experiments in liver-chimeric mice where mice were injected intravenously (IV) with 1 × 10(6) sporozoites on day 0 and then with human red blood cells (h) on days 5 and 6 prior to emergence of blood-stage parasites. Blood samples were taken on days 7 and 9 for qRT-PCR with day 9 samples used for 28-day in vitro culture of blood stages with subsequent parasitaemia readout by microscopy and qRT-PCR. b 18 S qPCR analysis of blood samples from FRG huHep mice on day 7 and 9 after infection with 1 × 10(6) sporozoites of PfΔmei2 (n = 7 mice) and WT PfNF54 (n = 4 mice). Significance values (unpaired Mann–Whitney test): **p < 0.001. Dotted line: the cutoff as used in controlled human malaria infection (CHMI) at OHSU of 5 parasites/ml, assuming 7400 18 S copies/per parasite. c Analysis of blood samples from FRG huHep mice for presence of blood-stage parasites by in vitro cultivation of blood stages. Cultures, maintained in a semi-automated shaker system, were monitored for blood-stages for 28 days by microscopy analysis of Giemsa-stained thin and thick blood smears (see d) and by 18 S qPCR. Significance values (unpaired Mann–Whitney test): **p < 0.001. Dotted line: the 10 parasites/ml cutoff used in CHMI at LUMC, assuming 4252 18 S copies per parasite. d Blood-stage parasites in cultured blood samples collected from FRG huHep mice (m) after infection with PfΔmei2 and WT PfNF54 sporozoites. Samples from in vitro cultures were analyzed at different days (d) for blood-stage parasitemia by microscopy (% of infected RBC).