Figure 11. Simplified schemes illustrating chaperone-assisted protein folding.
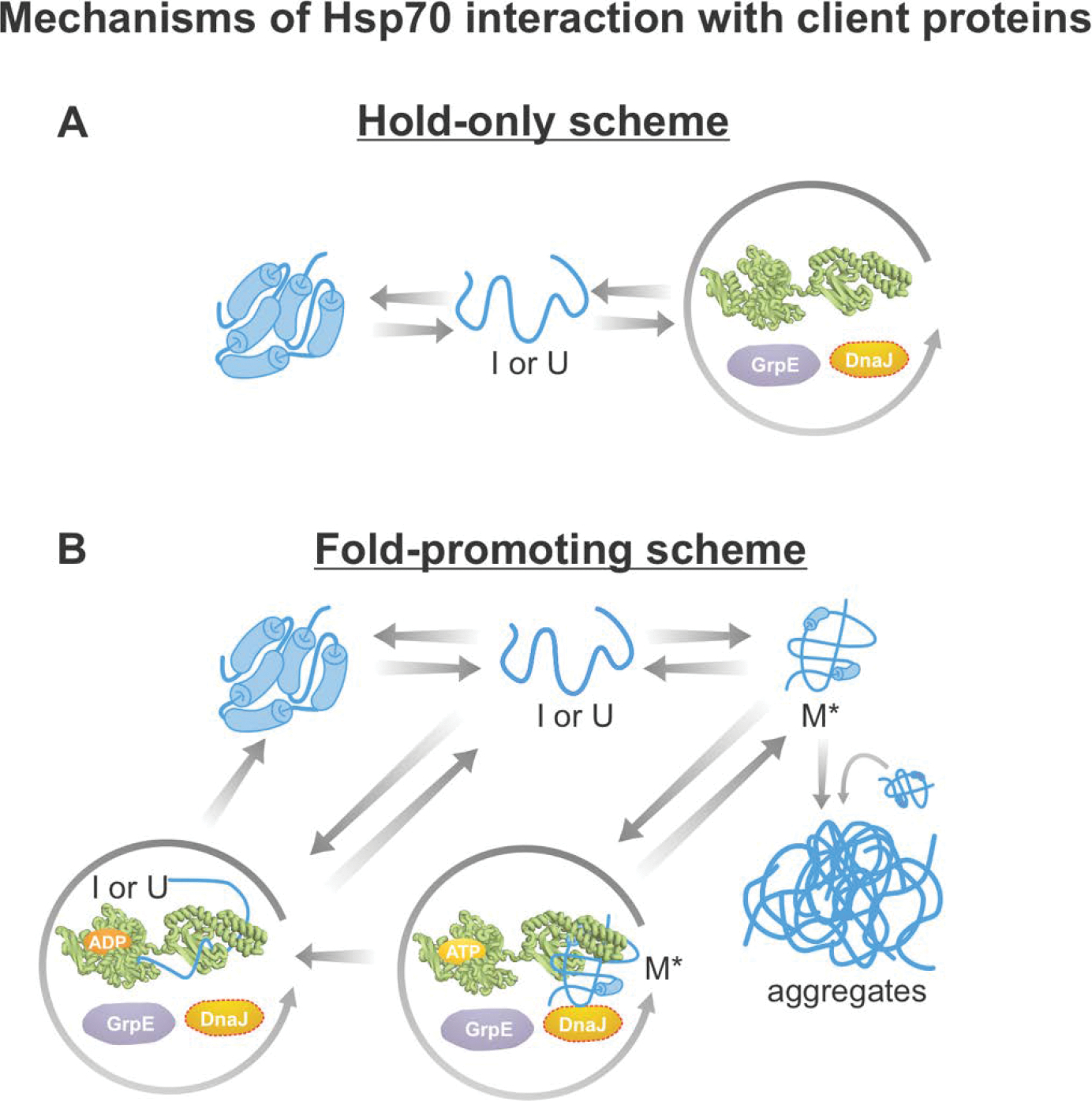
The diagrams in this figure are consistent with experimental results achieved with distinct classes of client proteins. (A) Hold-only model consistent with both computational and experimental results on non-aggregation-prone proteins bearing one Hsp70 binding site [307, 315]. (B) Fold-promoting models consistent with experimental results obtained with aggregation-prone client proteins bearing multiple chaperone binding sites per molecule. For instance, firefly luciferase (fluc) populate their native states more quickly and avoid generating aggregates in the presence of the Hsp70 chaperone system [259]. According to this fold-promoting model, the Hsp70 chaperone system catalyzes the conversion of misfolded monomers (M*) to the native state and, in so doing, increases the yields and observed rates of native-structure formation.
