Figure 5. Representative protein folding energy landscapes.
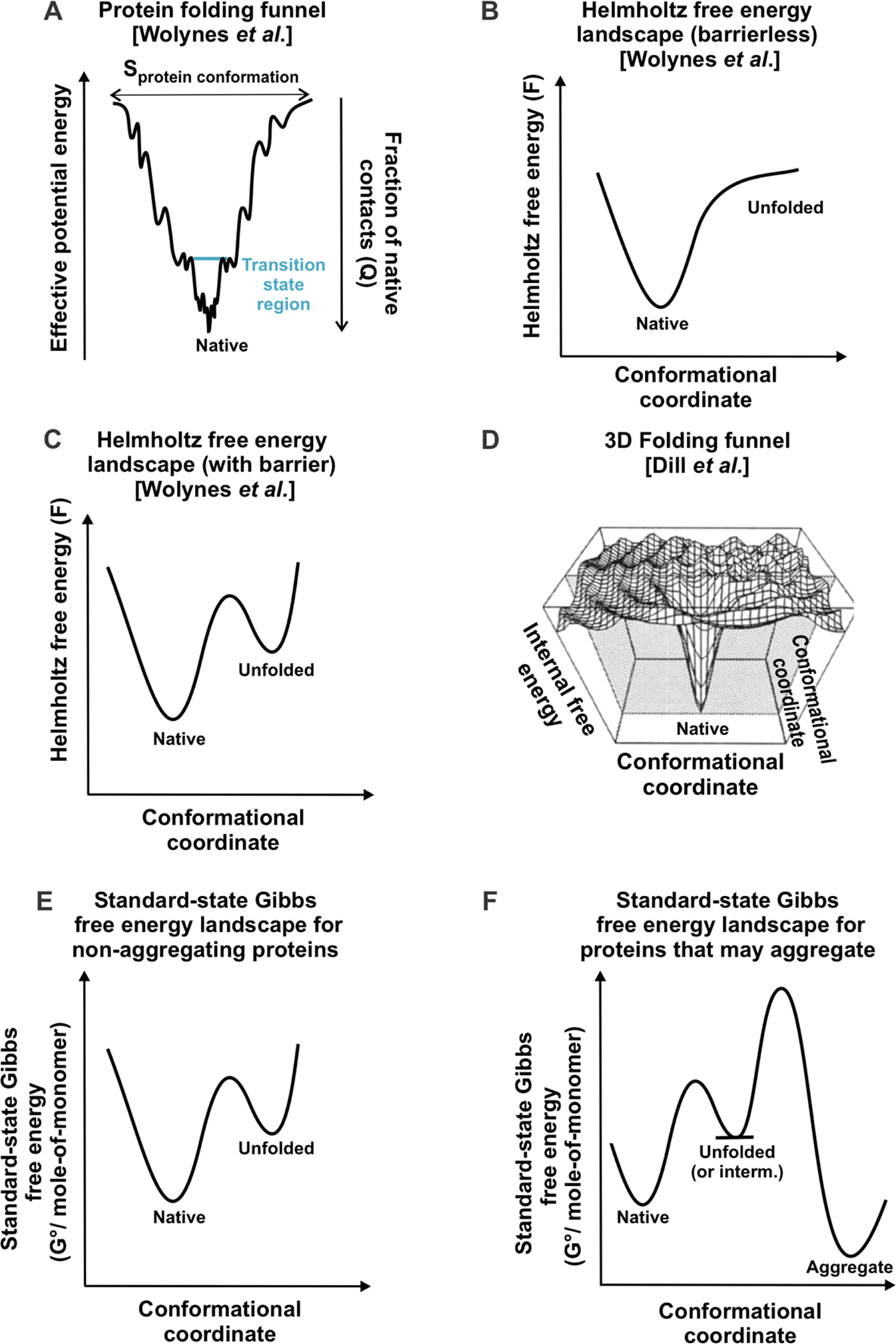
(A) Folding funnel proposed by Wolynes and coworkers, showing how protein conformational entropy decreases in concert with effective potential energy, as a protein folds to its native state [104, 116]. (B) Helmholtz free energy landscape for proteins that do not have a free-energy transition state for folding. (C) Helmholtz free energy landscape for proteins that have a free-energy transition state for folding. In panels A-C, the native state has 100% native contacts (Q = 1), and the unfolded state has Q = 0. (D) Multidimensional energy landscape. The vertical axis represents the potential energy of any given protein conformation plus the free energy of solvation [113]. Figure 1D is reprinted with permission from John Wiley and Sons [41] from figure 37C in Protein Science 4, Dill, K. A.; Bromberg, S.; Yue, K.; Chan, H. S.; Ftebig, K. M.; Yee, D. P.; Thomas, P. D. Principles of Protein Folding — a Perspective from Simple Exact Models. 4, 561–602. Copyright (1995). (E) Standard-state Gibbs free energy landscape of a protein that cannot form aggregates at a given temperature, pressure and solution conditions. (F) Gibbs free energy landscape for a protein that can form aggregates at a given temperature, pressure and solution conditions.
