Figure 8. Protein folding in the presence of the trigger factor (TF) chaperone.
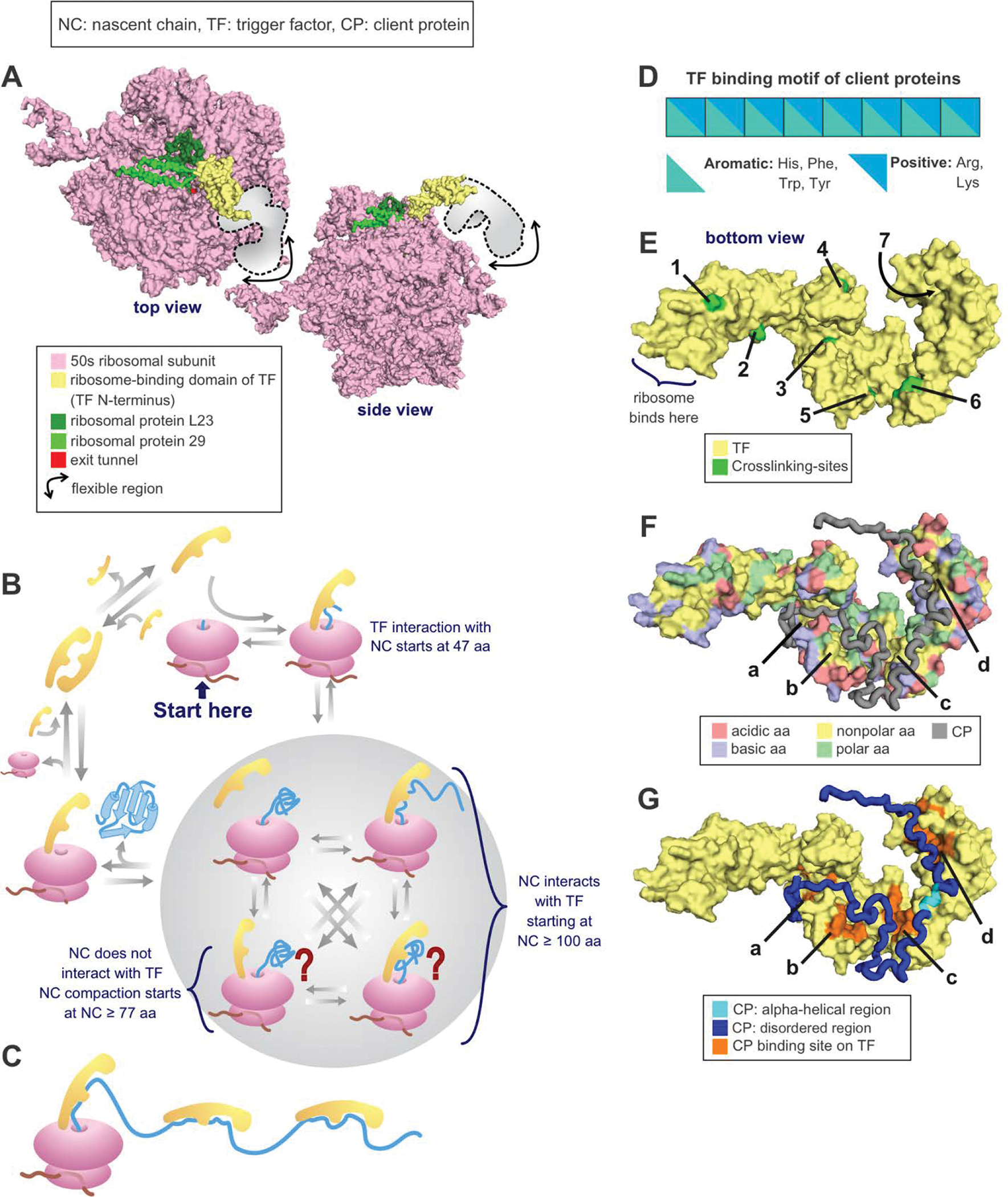
(A) Crystal structure of the RBD of TF bound to the 50s unit of the ribosome from eubacterium Deinococcus radiodurans. PDB: 2AAR [223]. (B) TF cycle. Note that TF is in rapid equilibrium with the ribosome. (C) Multiple TFs can be associated with the same nascent chain during translation or with the client protein in solution. (D) Nascent chain binding site for TF. (E) Crosslinking sites used to track the progression of the nascent chain as it travels throughout the TF [220]. PDB: 2MLX. (F) E. coli TF amino acids (aa) highlighted according to type. Note that the TF binding sites for PhoA, shown in the next panel, are all either nonpolar or polar. PDB: 2MLX. (G) E. coli TF associated with the 220–310 fragment of the PhoA client protein. PDB: 2MLX (Abbreviations: NC = nascent chain, TF = trigger factor, residues = amino acids).
