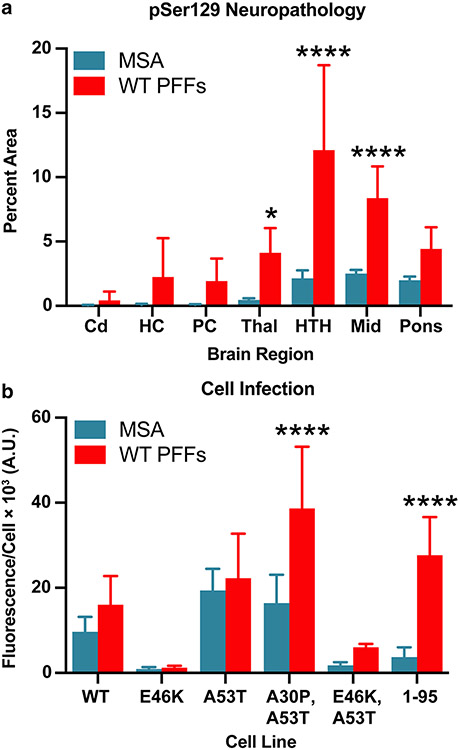
Fig. 7.
MSA and WT PFFs contain distinct α-synuclein prion strains. Eight-week-old TgM20+/− mice were inoculated with either MSA patient samples or recombinant WT PFFs. Measurements from MSA-inoculated mice were averaged across experiments testing all 6 MSA patient samples. a Quantification of stained brain slices showed a significant increase in phosphorylated α-synuclein lesions in the thalamus (Thal), hypothalamus (HTH), and midbrain (Mid). Cd caudate, HC hippocampus, and PC piriform cortex and amygdala (* = P < 0.05; **** = P < 0.0001). b Brain homogenates were assayed for α-synuclein prion transmission using our cell-based assay [35, 39]. α-Synuclein prions were isolated from brain homogenates via phosphotungstic acid precipitation and were incubated with α-syn–YFP cells for 4 d. The cell lines tested express wild-type (WT) α-syn–YFP, single mutations (E46K and A53T), double mutations (A30P,A53T and E46K,A53T), or A53T truncated at residue 95 (1–95). WT PFF-inoculated mouse samples showed greater infectivity in the A30P,A53T and 1–95 cell lines than the MSA-inoculated samples (**** = P < 0.0001)